Published online Jul 1, 2004. doi: 10.3748/wjg.v10.i13.1923
Revised: December 23, 2002
Accepted: December 30, 2002
Published online: July 1, 2004
AIM: To determine the role and effect of nitric oxide synthase type II (NOS II) in cirrhotic rats.
METHODS: Expression of NOS II mRNA was detected by real time RT-PCR. The activity of nitric oxide synthase and serum levels of NO, systemic and portal hemodynamics and degrees of cirrhosis were measured with high sensitive methods. Chinese traditional medicine tetrandrine was used to treat cirrhotic rats and to evaluate the function of NO. Double-blind method was applied during the experiment.
RESULTS: The concentration of NO and the activity of NOS were increased markedly at all stages of cirrhosis, and iNOSmRNA was greatly expressed. Meanwhile the portal-venous-pressure (PVP), and portal-venous-flow (PVF) were significantly increased. NO, NOS and iNOSmRNA were positively correlated to the quantity of hepatic fibrosis. Tetrandrine significantly inhibited NO production and the expression of iNOSmRNA.
CONCLUSION: Increased hepatic expression of NOS II is one of the important causes of hepatic cirrhosis and portal hypertension.
- Citation: Wang H, Chen XP, Qiu FZ. Increased hepatic expression of nitric oxide synthase type II in cirrhotic rats. World J Gastroenterol 2004; 10(13): 1923-1927
- URL: https://www.wjgnet.com/1007-9327/full/v10/i13/1923.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i13.1923
Nitric oxide (NO) is a vasodilator formerly detected in vascular system, but is currently recognized as a multifunctional molecule widely distributed in many other tissues, such as immune and neuronal systems. NO is synthesized from L-arginine by the NO synthase isozyme family of which three different members have been characterized, namely NOS I, NOS II and NOS III. NOS I and NOS III are constitutive and Ca2+/calmodulin-independent. The first was initially discovered in neurons and the second in endothelial cells[1-2]. NOS II is Ca2+-independent, and is located in macrophages and smooth muscle cells, and is induced by appropriate proinflammatory stimuli and bacterial lipolysaccharides[3]. Its activity can be competitively inhibited by several L-arginine analogues such as Nw-nitro-L-arginine.
However, proinflammatory cytokines, endotoxins, and bacterial infections in various pathologic entities are associated with enhanced production of NO, hyporesponsiveness to vasoconstrictors, and development of a hyperdynamic state[4-7]. Vallance and Moncada hypothesized that bacterial endotoxin might account for hemodynamic changes in severe cirrhosis by stimulating the expression of inducible NO synthase (iNOS) to produce NO[8].
Recently, numerous studies have focused on NO as a possible major causative agent of the decreased vascular resistance in cirrhosis. In fact, most investigations are coincident in reporting an increased NO activity in the vasculature of patients and rats with cirrhosis[9-12], a phenomenon in which both NOS II and NOS III seem to be involved[13]. There is, however, little information on the progress in liver diseases affecting the L-arginine/NO pathway in organs other than the systemic vasculature. This is particularly intriguing in the liver, as it largely affects the consequence of the derangement under pathologic conditions. Whereas most investigations consider NO as an important mediator of hemodynamic alterations in experimental cirrhosis[14].
Chinese traditional medicine tetrandrine has the function of inhibiting calcium influx[15,16], and effect in treatment of liver fibrosis[17]. We postulated that if NO was a key mediator in cirrhosis and portal hypertension, Tetrandrine would affect the level of NO by inhibiting the expression of NOSmRNA and the activity of NOS. Therefore, in this study, mRNAs of NOS II and NOS III were determined in liver tissue of rats with CCl4-induced cirrhosis to assess the level of NO. In addition, the NOS activity of liver tissue and the serum levels of NO, hemodynamic changes of portal vein and pathologic changes at all stages of cirrhosis were also investigated.
Hepatic injury and fibrosis were induced in male retired breeder Sprague-Dawley rats (450-550 g) by intragastric administration of carbon tetrachloride as described previously[18]. CCl4(1.0 mL/kg) in a 1:1 mixture with corn oil was administered by gavage at 7-day intervals. After the second intragastric administration of carbon tetrachloride, Tetrandrine group inhaled the suspension of Tetrandrine (1 mL/0.1 kg). Animal weight was monitored, and the dosage of CCl4 and Tetrandrine was adjusted accordingly. Portal hypertension (portal venous dilation with portal pressure > 10 cm H2O) was documented in all animals after 10 doses of CCl4. Controls received corn oil on the same schedule as experimental animals.
There were 90 conscious rats in this study. Animals were anesthetized with ketamine (100 mg/kg.bm) and prepared with P-50 catheters in portal vein and inferior vena cava. The catheter was connected to a highly sensitive transducer and a multichannel recorder (MX4P and MT4, Lectromed Ltd, Jersey, Chanels Islands, UK) and portal venous pressure and portal venous flow were recorded. Then, a 2-mL blood sample was taken to measure NO. Blood was immediately centrifuged at 4 °C and serum was frozen at -20 °C until further analysis. Tissue specimens were obtained from the middle liver lobe in each animal. Liver specimens were fixed in 40 g/L buffered formaldehyde and stained with hematoxylin and eosin, reticulin, and Masson’s trichrome for histological examination. Moreover, additional liver tissues were immediately frozen in dry ice and stored in liquid nitrogen until further analysis. Serum NO and liver NOS activity were determined.
mRNAs of NOS II and NOS III were assessed in liver tissues of all groups. Under anesthesia with ketamine (100 mg/kg) animals were exanguinated by cardiac puncture. Liver tissue was dissected, placed in a Petri dish containing a diethyl pyrocarbonate (0.01%)-treated PBS buffer solution, total RNA was extracted by the guanidine isothiocyanate method[19].
Reverse transcription (RT) reaction was performed with cDNA synthesis kit (Promega, Madison, WI) and followed the attached protocol with reaction kit. The final volume of the reaction (20 μL) was completed with RNase free water. First strand cDNA synthesis was carried out at 42 °C for 30 min in a DNA thermal cycler (PTC-100, MJ Reserch Inc, Watertown, MA). Afterwards, the tubes were incubated at 99 °C for 5 min to terminate the reaction. Then each tube was kept at 4 °C until PCR was performed.
Expression of the target genes (NOS II and NOS III) was quantified. The primers were designed using the software Primer Express (Applied Biosystems) (Table 1).
| Gene | Quantification method | Primer sequence | cDNA |
| NOS II | Forward primer | 5’-CCCTTCCGAAGTTTCTGGCAGCAG-3’ | 2793-2817 |
| Reverse primer | 5’-GGGCTCCTCCAAGGTGTTGCCC-3’ | 3424-3446 | |
| Probe | 5’-(FAM)TCTTCGGTGCGGTCTTTTCCTATGGAGCAA(TAMRA)-3’ | 3391-3421 | |
| NOS III | Forward primer | 5’-CAAGACCGATTACACGACAT-3’ | 31-51 |
| Reverse primer | 5’-GCTGGTTGCCATAGTGACAT-3’ | 300-321 | |
| Probe | 5’-(FAM)GAGTGTTTGGACAAGTCCTCACCGCCTTTT-(TAMRA)-3’ | 158-188 | |
| GAPDH | Forward primer | 5’-GAAGGTGAAGGTCGGAGTCA-3’ | |
| Reverse primer | 5’-GAAGATGGTGATGGGA-3’ | ||
| Probe | 5’-(JOE)CAAGCTTCCCGTTCTCAGCC(TAMRA)-3’ |
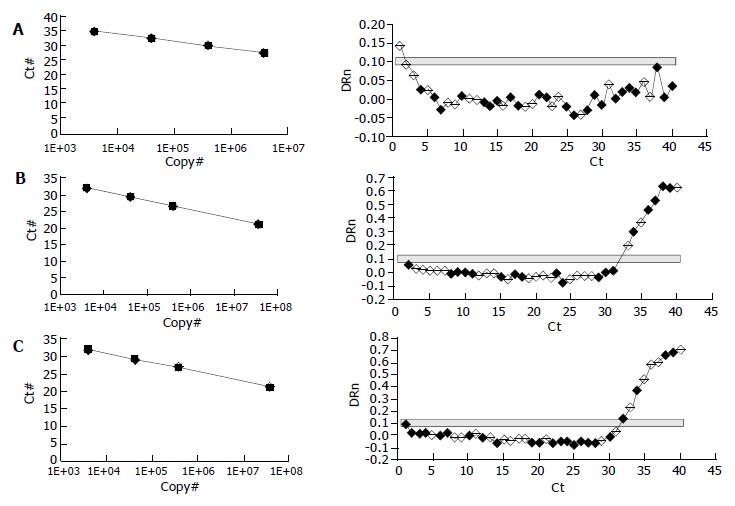
RT- PCR was performed according to a TaqMan 2-step method using an ABI PRISM 7700 sequence detection system (Applied Biosystems). PCR cycling conditions included an initial phase at 50 °C for 2 min, followed by at 95 °C for 10 min for AmpErase, 40 cycles at 95 °C for 15 s, and at 60 °C for 1 min. Quantification of the PCR products was based on the TaqMan 5' nuclease assay using the ABI PRISM 7700 sequence detection system. The starting amount of a specific mRNA in an unknown sample was determined. The standard curve was generated on the basis of the linear relationship between the Ct value and logarithm of the starting quantity. Ct value was the cycle number at which a significant increase in the fluorescence signal was initially detected. The unknown samples were quantified by the software of the ABI PRISM 7700 sequence detector system, which calculated the Ct value for each sample and then determined the initial amount of the target using the standard curve. The amount of the target was normalized to the reference.
One-way ANOVA, Newman-Keuls’s, and unpaired Student’s t tests were used for statistical analysis. Data are expressed as mean ± SE, P level at 0.05 or less was considered statistically significant.
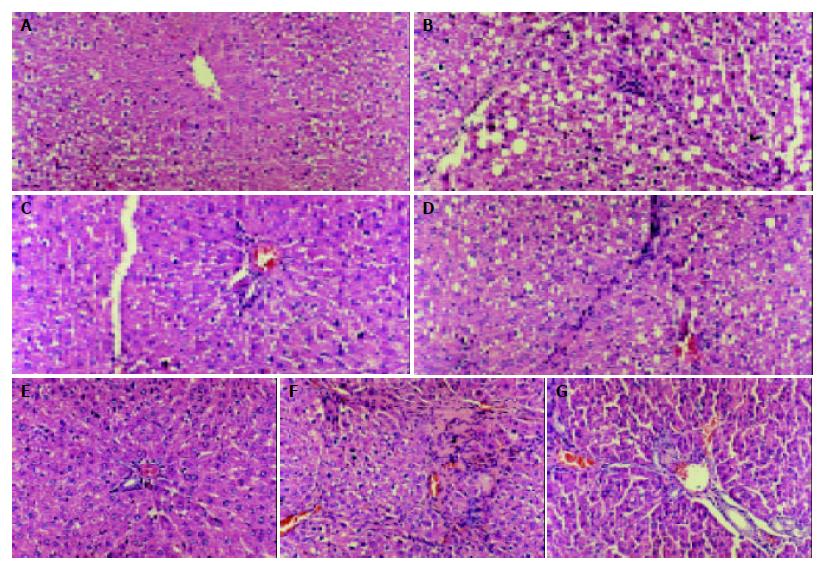
The cirrhotic liver treated with CCl4 had a finely granulated surface. Histological examination showed the distinct features of three-grade fibrosis. Tetrandrine group had slight fibrosis in the third grade of cirrhosis.
Pathologic examination indicated that tetrandrine had an obvious effect on preventing the necrosis of hepatic cells and delaying the development of hepatic fibrosis (Figure 1).
Serum NO and NOS activity in cirrhotic group were significantly higher than those in normal control, and had no difference between the normal and therapeutic groups (Table 2, Table 3).
High hemodynamics was observed in cirrhotic rats. Portal-venous-pressure (PVP) and portal-venous-flow (PVF) were significantly increased. But PVP in tetrandrine groups was significantly decreased in all stages of cirrhosis. PVF was only decreased in the metaphase of cirrhosis (Table 4).
| Stage | Index | Tetrandrine | Cirrhosis | Negative control |
| (n = 8) | (n = 8) | (n = 8) | ||
| Earlier period | PVF | 18.37 ± 1.83 | 21.25 ± 1.76 | 16.50 ± 1.73 |
| PVP | 1.35 ± 0.25b | 1.67 ± 0.21 | 1.18 ± 0.13 | |
| ICVP | 0.31 ± 0.04 | 0.32 ± 0.04 | 0.31 ± 0.04 | |
| PVR | 5.73 ± 0.88 | 6.38 ± 1.12 | 5.81 ± 1.04 | |
| Metaphase | PVF | 14.50 ± 1.61a | 20.35 ± 1.07 | 17.75 ± 1.37 |
| PVP | 1.44 ± 0.13b | 1.72 ± 0.69 | 1.30 ± 0.21 | |
| ICVP | 0.32 ± 0.04 | 0.31 ± 0.04 | 0.32 ± 0.04 | |
| PVR | 7.98 ± 1.68 | 9.56 ± 1.29 | 5.53 ± 0.67 | |
| Late period | PVF | 15.85 ± 1.23 | 17.57 ± 1.93 | 15.42 ± 1.27 |
| PVP | 1.45 ± 0.24b | 1.67 ± 0.23 | 1.37 ± 0.10 | |
| ICVP | 0.31 ± 0.04 | 0.32 ± 0.04 | 0.32 ± 0.04 | |
| PVR | 7.44 ± 1.08 | 8.01 ± 0.79 | 6.76 ± 0.34 |
NO concentration correlated significantly with PVP (r = 0.69, P < 0.01) and PVF (r = 0.72, P < 0.01), but inversely with PVR (r = -0.63, P < 0.01).
Quantification of the PCR products showed that NOS II was highly expressed in cirrhotic rat tissue (Figure 2C), and NOS II in therapeutic groups was significantly decreased than cirrhosis (Figure 2B) (P < 0.01). NOS II was not found in normal rat tissue (Figure 2A). NOS III expression was not different among all groups (P > 0.05) (Table 5).
| Stage | Index | Tetrandrine | Cirrhosis | Negative control |
| (n = 8) | (n = 8) | (n = 8) | ||
| Earlier period | NOS II | 0.1723 ± 0.0014 | 0.5635 ± 0.0104 | - |
| NOS III | 0.2578 ± 0.0076 | 0.2694 ± 0.0043 | 0.2786 ± 0.0107 | |
| Metaphase | NOS II | 0.1679 ± 0.0093 | 0.7521 ± 0.0098 | - |
| NOS III | 0.3210 ± 0.0084 | 0.3412 ± 0.0113 | 0.2916 ± 0.0089 | |
| Late period | NOS II | 0.1876 ± 0.0065 | 0.6987 ± 0.0078 | - |
| NOS III | 0.2917 ± 0.0102 | 0.3002 ± 0.0091 | 0.2815 ± 0.0098 |
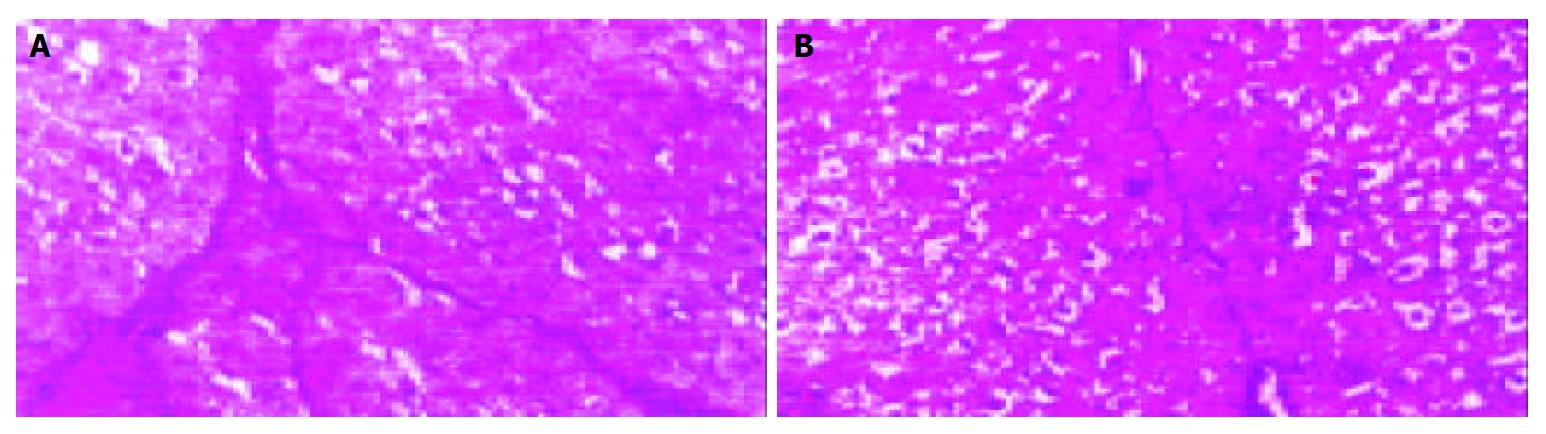
Quantification of cirrhosis showed that cirrhosis significantly decreased in Tetrandrine group than in cirrhotic group (Figure 3).
The function of NO in human beings or experimental animals is invariably associated with marked systemic and portal venous hemodynamic effects, including arterial hypotension, portal hypertension, increased portal venous perfusion. The intensity and full manifestation of this response depend on the expression of NOS and the NOS activity. To ascertain which isoforms of NOS displayed a differential effect on cirrhotic liver of rats, the expressions of NOS II and NOS III were determined. The study showed that NOS II was markedly increased in cirrhotic rat liver tissues. However, we did not find the expression of NOS II in normal liver tissues. Because macrophages were of central importance in the initiation and regulation of nonspecific and specific immune responses[20], it was considered that NO from these cells was implicated in the nonspecific defense against parasitic diseases and participated in the production of free radicals that are toxic to bacteria and parasites[21]. Rodent macrophages were extremely sensitive to agents promoting NOS II expression[22]. In this study, no difference in NOS III expression between cirrhotic and normal rats was found. We postulated that NOS III expression in cirrhotic liver had no effect on portal hypertension. But it might have a great function in cirrhosis due to its expression in big blood vessels[23-25]. Intrahepatic endothelial NO mediated by shear stress-dependence seems impaired by shunt.
Chinese traditional medicine Tetrandrine had a good therapeutic effect for fibrosis[16,17]. It is often used to treat hepatitis of HBV. In a recent study, it was tested to inhibit calcium influx. We therefore postulated that if NO played a role in the mechanism of cirrhosis and portal hypertension, Tetrandrine would have the effect on the inhibition of NO production. This study approved our hypothesis and showed NOS II mRNA was markedly expressed in cirrhotic liver tissues. NOS III was mainly distributed in endothelial cells and its function was relied on calmodulin(CaM)[26]. Tetrandrine affected NOS III expression, however, had no effect on NOS III expression. Thus, we reason the changes of NOS III could be stimulated by shear stress. NOS II was mainly distributed in microphages and endothelial cells. NOS II did not express in normal physiological conditions. Amino acid sequence analysis showed that NOS II had the conjugated site of calmodulin, and rodent NOS II could be conjugated tightly with CaM without calcium[27]. In this study, an important finding in cirrhotic liver exposed to Tetrandrine was an NO antagonist. Moreover, tetrandrine could also affect NOS enzymatic activity. The results of the present study indicated that portal-venous-pressure and portal-venous-flow decreased by tetrandrine were mediated by lower level NO and cirrhotic blood vessel.
In conclusion, NOS II is markedly expressed in cirrhotic liver, inducing a high hemodynamic change of portal vein and pathologic changes in all stages of cirrhosis. Tetrandrine inhibits NOS II expression, decreases NOS activity and reduces the NO production. The mechanism of fibrosis and portal hypertension is NO mediated, and tetrandrine is effective for improving portal hypertension by reducing NO in cirrhotic patients.
The authors are acknowledged the skillful technical assistance of Dr. Bing-Quan Wu.
| 1. | Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991;351:714-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1833] [Cited by in RCA: 1781] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 2. | Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci USA. 1992;89:6348-6352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 646] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 3. | Lowenstein CJ, Glatt CS, Bredt DS, Snyder SH. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci USA. 1992;89:6711-6715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 426] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Guarner C, Soriano G, Tomas A, Bulbena O, Novella MT, Balanzo J, Vilardell F, Mourelle M, Moncada S. Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology. 1993;18:1139-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 290] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Lee FY, Wang SS, Yang MC, Tsai YT, Wu SL, Lu RH, Chan CY, Lee SD. Role of endotoxaemia in hyperdynamic circulation in rats with extrahepatic or intrahepatic portal hypertension. J Gastroenterol Hepatol. 1996;11:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 271] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide. Lancet. 1991;337:776-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 417] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Clària J, Jiménez W, Ros J, Asbert M, Castro A, Arroyo V, Rivera F, Rodés J. Pathogenesis of arterial hypotension in cirrhotic rats with ascites: role of endogenous nitric oxide. Hepatology. 1992;15:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Ros J, Jiménez W, Lamas S, Clària J, Arroyo V, Rivera F, Rodés J. Nitric oxide production in arterial vessels of cirrhotic rats. Hepatology. 1995;21:554-560. [PubMed] |
| 11. | Sieber CC, Lopez-Talavera JC, Groszmann RJ. Role of nitric oxide in the in vitro splanchnic vascular hyporeactivity in ascitic cirrhotic rats. Gastroenterology. 1993;104:1750-1754. [PubMed] |
| 12. | Morales-Ruiz M, Jiménez W, Pérez-Sala D, Ros J, Leivas A, Lamas S, Rivera F, Arroyo V. Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites. Hepatology. 1996;24:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 82] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Martin PY, Xu DL, Niederberger M, Weigert A, Tsai P, St John J, Gines P, Schrier RW. Upregulation of endothelial constitutive NOS: a major role in the increased NO production in cirrhotic rats. Am J Physiol. 1996;270:F494-F499. [PubMed] |
| 14. | Ros J, Clària J, Jiménez W, Bosch-Marcé M, Angeli P, Arroyo V, Rivera F, Rodés J. Role of nitric oxide and prostacyclin in the control of renal perfusion in experimental cirrhosis. Hepatology. 1995;22:915-920. [PubMed] |
| 15. | Liu YL, Li DG, Lu HM, Xu QF. The control to 3T6 fibroblast from four calcium antagonist. Beijing Yixue Zazhi. 1996;18:26-29. |
| 16. | Wang BE, Sun M, Bai N, Li XM, Yin WY, Wang TL. The thera-peutic observation to experimental liver fibrosis of the active and decongestive Chinese Medicine. Zhongcaoyao. 1990;21:23. |
| 17. | Li DG, Liu YL, Lu HM, Jiang ZM, Xu QF. The effects of tetrandrine to mitochondrion of cirrhotic rat. Zhonghua Xiaohua Zazhi. 1994;14:339-342. |
| 18. | Andiran F, Kilinç K, Renda N, Ayhan A, Tanyel FC. Lipid peroxidation and extracellular matrix in normal and cirrhotic rat livers following 70% hepatectomy. Hepatogastroenterology. 2003;50:805-808. [PubMed] |
| 19. | Kimura B, Kawasaki S, Nakano H, Fujii T. Rapid, quantitative PCR monitoring of growth of Clostridium botulinum type E in modified-atmosphere-packaged fish. Appl Environ Microbiol. 2001;67:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Kincaid EZ, Ernst JD. Mycobacterium tuberculosis exerts gene-selective inhibition of transcriptional responses to IFN-gamma without inhibiting STAT1 function. J Immunol. 2003;171:2042-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3127] [Cited by in RCA: 3100] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 22. | Lyons CR, Orloff GJ, Cunningham JM. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992;267:6370-6374. [PubMed] |
| 23. | Cahill PA, Redmond EM, Hodges R, Zhang S, Sitzmann JV. Increased endothelial nitric oxide synthase activity in the hyperemic vessels of portal hypertensive rats. J Hepatol. 1996;25:370-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Gadano AC, Sogni P, Yang S, Cailmail S, Moreau R, Nepveux P, Couturier D, Lebrec D. Endothelial calcium-calmodulin dependent nitric oxide synthase in the in vitro vascular hyporeactivity of portal hypertensive rats. J Hepatol. 1997;26:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Martin PY, Xu DL, Niederberger M, Weigert A, Tsai P, St John J, Gines P, Schrier RW. Upregulation of endothelial constitutive NOS: a major role in the increased NO production in cirrhotic rats. Am J Physiol. 1996;270:F494-F499. [PubMed] |
| 26. | Murthy KS, Zhang KM, Jin JG, Grider JR, Makhlouf GM. VIP-mediated G protein-coupled Ca2+ influx activates a constitutive NOS in dispersed gastric muscle cells. Am J Physiol. 1993;265:G660-G671. [PubMed] |
| 27. | Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1992;176:599-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 433] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
Edited by Ren SY and Wang XL Proofread by Xu FM