Published online Jul 1, 2004. doi: 10.3748/wjg.v10.i13.1918
Revised: January 10, 2004
Accepted: January 17, 2004
Published online: July 1, 2004
AIM: Cyclooxygenases (COX) are key enzymes for conversion of arachidonic acid to prostaglandins. Nitric oxide synthase (NOS) is the enzyme responsible for formation of nitric oxide. Both have constitutive and inducible isoforms. The inducible isoforms (iNOS and COX-2) are of great interest as regulators of tumor angiogenesis, tumorigenesis and inflammatory processes. This study was to clarify their role in pancreatic adenocarcinomas.
METHODS: We investigated the immunohistochemical iNOS and COX-2 expression in 40 pancreatic ductal adenocarcinomas of different grade and stage. The results were compared with microvessel density and clinicopathological data.
RESULTS: Twenty-one (52.5%) of the cases showed iNOS expression, 15 (37.5%) of the cases were positive for COX-2. The immunoreaction was heterogeneously distributed within the tumors. Staining intensity was different between the tumors. No correlation between iNOS and COX-2 expression was seen. There was no relationship with microvessel density. However, iNOS positive tumors developed more often distant metastases and the more malignant tumors showed a higher COX-2 expression. There was no correlation with other clinicopathological data.
CONCLUSION: Approximately half of the cases expressed iNOS and COX-2. These two enzymes do not seem to be the key step in angiogenesis or carcinogenesis of pancreatic adenocarcinomas. Due to a low prevalence of COX-2 expression, chemoprevention of pancreatic carcinomas by COX-2 inhibitors can only achieve a limited success.
- Citation: Kasper HU, Wolf H, Drebber U, Wolf HK, Kern MA. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in pancreatic adenocarcinoma: Correlation with microvessel density. World J Gastroenterol 2004; 10(13): 1918-1922
- URL: https://www.wjgnet.com/1007-9327/full/v10/i13/1918.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i13.1918
Cancer of the pancreas is still one of the most dismal malignant diseases with a death to incidence rate of 0.99[1]. In Germany alone, more than 11000 patients die of this disease per year[2]. The only effective therapy is surgical excision[3]. Adjuvant chemo- and radiotherapy provide only a minimal survival advantage without consistent improvement in outcome[4]. Until now, the molecular biology of pancreatic cancer still is relatively unknown. A number of studies have focused on genetic changes[3,5,6]. But also inflammation has been identified as a significant factor in the development of pancreatic cancer[7]. Both hereditary and sporadic forms of chronic pancreatitis may be associated with increased cancer risk[8]. Cytokines, reactive oxygen species and mediators of the inflammatory pathway have been identified to increase cell cycling, cause loss of tumor suppressor function and stimulate oncogene expression[7]. These cytokines are also part of angiogenesis, being an important step for tumor development and could be a therapeutic target also for pancreatic adenocarcinomas.
Nitric oxide (NO) is a pleiotropic biomolecule with a short half time. It has part in the signal transduction in a great variety of mechanisms including neural transmission, vasodilatation, immunoregulation and defense mechanism as well as influencing cancerogenesis[9]. NO is a product of the conversion of l-arginine to l-citrulline by nitric oxide synthase (NOS)[10]. This enzyme exists in three isoforms, namely the constitutively expressed calcium-dependent endothelial (eNOS) and neuronal (nNOS) nitric oxide synthase and the calcium independent inducible or immunological (iNOS or NOS-2) isoforms[11].
INOS expression in human diseases has long been a matter of investigation. NO production by iNOS in tumor infiltrating macrophages may be part of their antitumoral cytotoxic potential[12]. On the other hand, expression of iNOS in endothelial cells of tumor vessels and in cancer cells itself supports the assumption that the cancer uses this isoform to regulate the tumor vascularisation and blood flow[13,14].
Cyclooxygenases (COX) are enzymes which are involved in the synthesis of prostaglandines (PGs) from arachidonic acid. They catalyze the insertion of molecular oxygen into arachidonic acid to form the unstable intermediate PG-G2 being rapidly converted to PGH2. PGH2 is the source of several biological active PGs, thromboxanes and prostacyclines which contribute to many physiological and pathological processes like hemostasis, kidney and gastric function, pain, inflammation and tumor defense and also tumorigenesis[15].
The two isoforms of COX (COX-1 and COX-2) differ in many respects[16]. COX-1 is constitutively expressed in most tissues and seems to be responsible for the baseline production of PG. COX-2 is not detected in most normal tissues but is highly inducible by inflammatory and mitogenic stimuli. COX-2 is strongly implicated in tumorigenesis and COX-2 inhibitors are discussed as the target for cancer prevention and therapy[17].
Current knowledge regarding the rate of iNOS and COX-2 expressions and their relationship to MVD in pancreatic cancer is rather limited. We investigated therefore iNOS and COX-2 expression in pancreatic adenocarcinomas in comparison to microvessel density to clarify the influence of these mediators in pancreatic carcinomas.
All tissues used in this study were surgical resection specimens obtained from the files of the Department of Pathology, Johannes Gutenberg- University, Mainz, Germany. All pancreatic adenocarcinomas were of ductal origin. Carcinomas from 18 women and 22 men with a mean age of 62.2 years (range 45- 81 years) were investigated. Thirty-three tumors were located in the head of the pancreas, 3 in the corpus and 5 in the tail of the pancreas. TNM- classification according to the TNM classification of malignant tumors was as follows: 5 × T1, 27 × T2, 7 × T3, 1 × T4. Twenty-three patients showed lymph node metastases and in 5 patients, distant metastases were known. There were 3 well differentiated, 15 moderately differentiated and 22 poorly differentiated pancreatic carcinomas.
Resected specimens were fixed overnight in 40 g/L buffered formaldehyde, embedded in paraffin and further processed.
For iNOS detection, 4-µm thick sections were deparaffinized and rehydrated, washed in tap water and distilled water and finally in 0.05 mol/L Tris buffer (pH7.6). After blocking of nonspecific binding sites (Protein blocking agent, Ultratech, Coulter-Immunotech, Marseille, France), sections were washed and incubated with the primary polyclonal rabbit antibody (Transduction Laboratories, Biomol, Hamburg, Germany) with a dilution of 1:1200 overnight at 4 °C. This antibody is directed against the mouse iNOS C-terminal peptide (1131-1144) plus additional N-terminated Cys conjugated to KLH (CKKGSALEEPKATRL). According to the manufacturer, the antibody could recognize the human, mouse and rat iNOS 130 kDa protein without cross reaction to eNOS or nNOS. A biotinylated goat anti-rabbit antibody (Vectastain, ABC-AP Elite Kit, Vector Laboratories, Burlingame, USA) served as secondary antibody. After incubation with the avidine-biotin-complex with alkaline phosphatase, as described by the manufacturer, Fast Red chromogen system (Coulter Immunotech) was used for visualization.
For COX-2 immunohistochemistry, antigen retrieval was achieved by microwave treatment 3 times for 5 min with 600 watt in 0.01 mol/L citrate buffer pH6.0. The monoclonal antibody COX-2 (h-62 sc-7951;Santa Cruz, Santa Cruz, CA, USA) was used as primary antibody in a dilution of 1:50. According to the producer, this antibody reacts with COX-2 of mouse, rat and human origin with no cross reaction with Cox-1. For visualization, the envision kit (DAKO, Glostrup, Denmark) was used.
The results of MVD were retrieved from a previous study[18]. In brief, it was determined using a monoclonal mouse anti-CD34 antibody (Biogenex, San Ramon, Clif, USA). After the endogenous peroxidase was blocked with 30 mL/L hydrogen peroxide the staining procedure was automated including detection with DAB (Ventana, Strassbourg, France).
Nuclear counterstaining was done in all sections with hematoxyline. For every staining procedure sections were incubated without the primary antibody as negative controls. Tissues from colorectal cancer were used as positive controls for all three antibodies.
Semiquantitative analysis of the immunostainings for iNOS and COX-2 was performed for the whole tissue sections considering staining intensity and percentage of positive tumor cells. Using a light microscope (Aristoplan, Leitz, Wetzlar, Germany), in ten randomly selected tumor areas the amount of positive tumor cells and the amount of all tumor cells were determined using an ocular grid at 400 ×. A case without positive tumor cells was considered negative. A case with less than 10% positive tumor cells was scored 1, 10%-50% was scored 2, 50%-80% was scored 3 and more than 80% was scored 4. The staining intensity was divided in weak (I), moderate (II) and strong (III). An immunoreactive score was calculated by multiplication of staining intensity with the amount of positive cells (lowest end score 0, highest end score 12). This evaluation was performed according to Remmele[19]. Specimens with a grade of more than 1 were regarded as positive.
Intratumoral MVD was measured as previously described[18]. In brief, stained sections were screened at 50 × magnification to identify the highest vascular area within the tumor. In these hot spots, individual microvessel count was evaluated at 200 × magnification using an ocular grid. Every cluster of cells stained with CD34 was counted as a vessel. Branching structures were counted as single vessels. Vascular counts for each case were calculated.
Statistical analysis was performed with the Statistical Program for Social Science (SPSS, Chicago, Ill., USA). The Pearson’s chi-square test and Fischer’s exact test were used to examine the association with the clinicopathological parameters. Nonparametric correlation with Spearman’s rho correlation coefficient was used to compare iNOS and COX-2 expressions as well as MVD. A P-value < 0.05 was considered statistically significant.
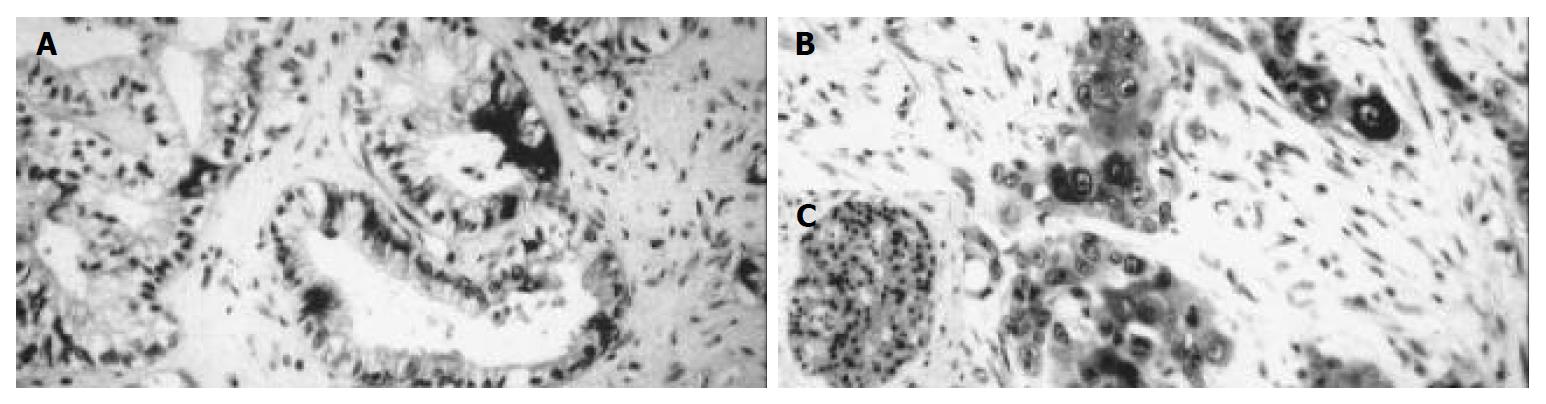
Positive iNOS immunostaining was detected in 21 (52.5%) of all 40 cases of pancreatic carcinoma. Immunoreactive tumor cells were both diffusely and focally distributed throughout the tumor. The immunoreaction was seen in the cytoplasm with completely unstained nuclei (Figure 1). Nine cases (22.5%) showed a mild staining intensity (grade I), 8 cases (20%) a moderate staining intensity (grade II) and 4 cases (10%) a strong staining intensity (grade III). One case (2.5%) expressed iNOS only focally in less than 10% of tumor cells. Eight cases (20%) showed immunoreaction in 10%-50% of tumor cells, 11 cases (27.5%) in 50%-80% of positive tumor cells. In one case (2.5%) more than 80% of tumor cells were stained (Table 1).
| Variables | No. of | INOS | INOS | P value | COX-2 | COX-2 | P value |
| patients (%) | positive | negative | positive | negative | |||
| Tumor stage | |||||||
| I | 5 (12.5) | 1 | 4 | 0.971 | 1 | 4 | 0.983 |
| II | 27 (67.5) | 15 | 12 | 11 | 16 | ||
| III | 7 (17.5) | 4 | 3 | 3 | 4 | ||
| IV | 1 (2.5) | 1 | 0 | 0 | 1 | ||
| Nodal stage | |||||||
| n = 0 | 17 (42.5) | 10 | 7 | 0.652 | 6 | 11 | 0.945 |
| n = 1 | 23 (57.5) | 11 | 12 | 9 | 14 | ||
| Distant metastases | |||||||
| M0 | 35 (87.5) | 18 | 17 | 0.043 | 13 | 22 | 0.459 |
| M1 | 5 (12.5) | 3 | 2 | 2 | 3 | ||
| Grade | |||||||
| G1 | 3 (7.5) | 2 | 1 | 0.591 | 1 | 2 | 0.000 |
| G2 | 15 (37.5) | 10 | 5 | 6 | 9 | ||
| G3 | 22 (55) | 9 | 13 | 8 | 14 | ||
| MVD | 5.7 (+/-2.4) | 6.4 (+/-2.9) | 6.4 (+/-3) | 5.8 (+/- 2.4) |
There was no correlation with tumor stage (P = 0.971), histological grade (P = 0.591), location within the pancreas (P = 0.184), lymph node metastases (P = 0.652), age (P = 0.651) or sex (P = 0.546) of the patients. A relationship between iNOS expression and distant metastases was seen, but the low amount of cases with distant metastases did not allow statistical evaluation.
In the surrounding tissue the islets of Langerhans showed positive reaction in several cells. The original acini were negative. In the negative controls, immunoreactivity was completely lacking while the positive controls displayed a clear cut immunopositivity.
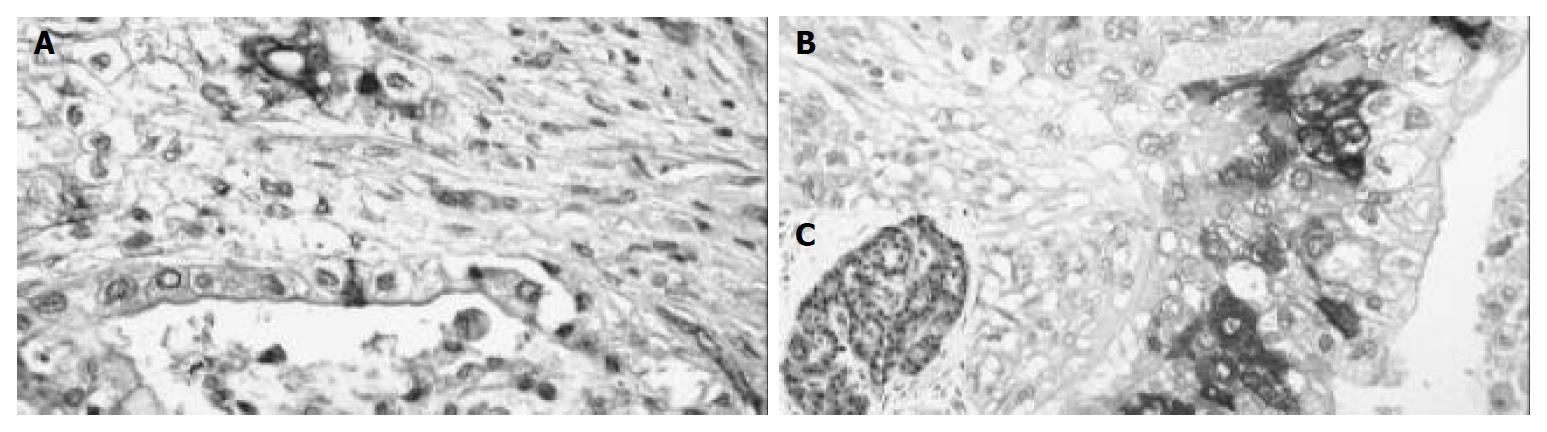
Positive COX-2 staining was seen in 15 out of the 41 (37.5%) pancreatic carcinomas. The immunoreactivity here was also focally distributed throughout the tumor with cytoplasmic reaction only. Nuclear staining was not observed (Figure 2). The staining intensity in the positive cases differed. Among them, 5 cases (12.5%) were slightly stained (grade I), 4 cases (10%) were moderately stained (grade II) and 6 cases (15%) were intensely stained (grade III). The amount of positive cells was as follows: 3 cases (7.5%) with less than 10% positive tumor cells, 7 cases (17.5%) with 10%-50% positive tumor cells, 4 cases (10%) with 50%-80% positive tumor cells and 1 case with more than 80% (2.5%) positive tumor cells (Table 1).
Comparing the immunoreactive score of COX-2 expression with histological grade, a significant relationship could be seen. The more malignant tumors (grade 3) showed a higher COX-2 expression (P = 0.000). This association depended stronger on the amount of positive cells (P = 0.000). Considering staining intensity alone, no relationship was seen (P = 0.118). The carcinomas in the head of the pancreas showed more often COX-2 expression than in other locations (P = 0.006).
COX-2 did not correlate with tumor stage (P = 0.983), lymph node (P = 0.945) or distance metastases (P = 0.459), age (P = 0.369) or sex (P = 0.331) of the patients.
In the surrounding tissue, the islets of Langerhans had positive reaction in several cells. The original acini were negative. In the negative control, immunoreactivity was completely lacking while the positive control displayed a clear cut immunopositivity.
The mean MVD in pancreatic carcinoma was 6.08 vessels per mm² (range 1.6 to 11.8 vessels per mm², standard derivation of 2.67 vessels per mm², median value of 5.25 vessels per mm²).
MVD analysis demonstrated a significant relationship to the age groups (younger or older than 60 years) with higher MVD in the older patients (P = 0.024). The was no statistically significant trend regarding the histological grade with more malignant carcinomas (grade 3) having higher MVD (P = 0.056).
MVD had no association with tumor stage (P = 0.154), location within the pancreas (P = 0.678), lymph node (P = 0.325) or distance metastases (P = 0.137) or sex (P = 1.000) of the patients.
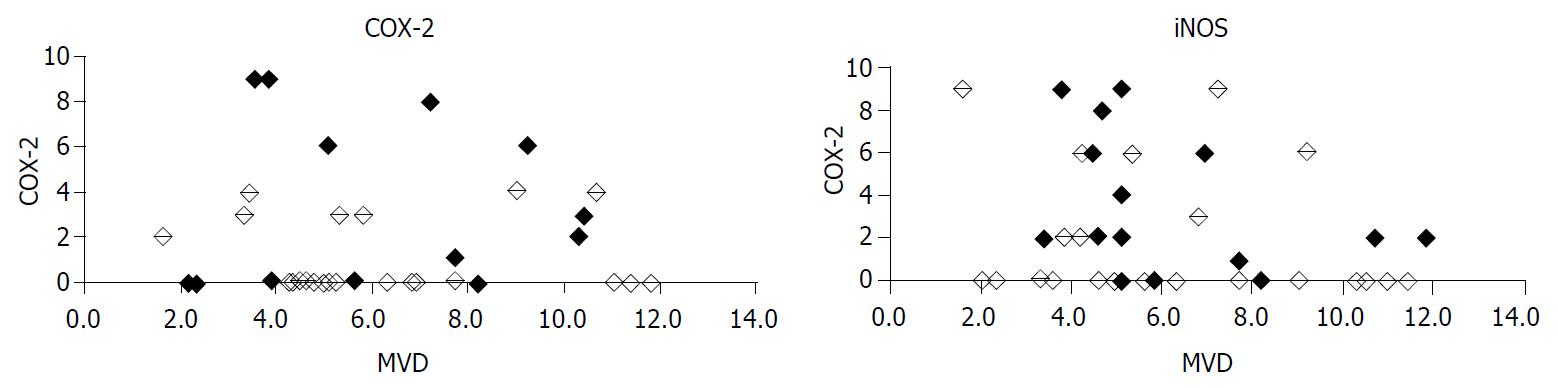
There was no statistically significant correlation between INOS and COX-2 expression in pancreatic adenocarcinomas (P = 0.137, Figure 3). MVD did neither depend on iNOS expression (P = 0.160) nor COX-2 expression (P = 0.989). Considering only COX-2 positive cases, no correlation of COX-2 with MVD could be found (P = 0.459). Also, considering iNOS positive cases alone, there was no relationship between iNOS expression and MVD (P = 0.436) noticeable.
Folkman et al[20] suggested that the step from prevascular to vascular phase was the prerequisite for the growth and spread of many solid tumors. Thus, angiogenesis contributes significantly to the progression of carcinomas. Intratumoral microvessel density is believed to reflect the overall degree of tumor angiogenesis. It has been accepted as an independent prognostic factor for several types of tumors like breast cancer, colon cancer or lung adenocarcinomas[21-23]. For pancreatic carcinoma, we could not find a correlation with parameters linked to survival[18].
Both COX-2 and iNOS, were play- markers of tumor angiogenesis[24]. Hypoxia induces iNOS together with erythropoietin, the molecule leading to erythropoesis and improvement of oxygen supply. COX-1 could regulate angiogenesis in endothelial cells, whereas COX-2 could regulate the production of nearly all angiogenetic factors in cancer cells[24].
We could show that iNOS was inconstantly expressed in pancreatic adenocarcinomas. This is in concordance with other studies[25,26]. INOS expression has been observed also in other carcinomas such as urothelial carcinomas[27], gynecological cancers[28], breast carcinomas[29], colon cancers[14], and lung tumors[30].
The role of iNOS in cancerogenesis is still not clear. NO, the product of NOS, has several properties in addition to angiogenesis, which might enhance tumor growth. NO has been found to be a mediator of angiogenesis and blood flow[31]. Also, high concentrations of NO could lead to apoptosis, low concentrations might protect many cells from cell death[32-34]. It could be shown that NO induces a G1-arrest followed by apoptosis in pancreatic carcinoma cell lines[35].
Multiple lines of evidence suggest that COX-2 is important in carcinogenesis. For example, COX-2 was upregulated in various forms of cancers[36]. A null mutation for COX-2 caused marked reduction in intestinal polyposis in a murine model of familial adenomatous polyposis[37]. Newly developed selective inhibitors of COX-2 were reported to protect against gastrointestinal tumor formation.
We found COX-2 expression in 15 out of 40 cancer cases. This is slightly lower than that reported in the literature, which was up to 74% by using different methods including Northern and Western blot as well as immunohistochemistry[38-40]. Okami reported a weak staining in most of the investigated carcinoma specimens[41]. COX-2 staining in pancreatic carcinoma was very heterogeneous and there were great variations between specimens.
For pancreatic carcinoma, Koshiba et al[42] suggested that COX-2 might be associated with the degree of malignancy comparing intraductal papillary mucinous adenomas, intraductal papillary carcinomas and intraductal carcinomas. We and others could show that in the group of carcinomas the expression of COX-2 depended on the histological grading, and higher malignant tumors showed more enhanced COX-2 expression. A dependence of histological grade was also described by Niijima[43]. With a higher expression in more malignant tumors COX-2 seems to be a late or bystander effect. In our cohort, we could not prove a positive or negative correlation of COX-2 expression with MVD.
In chronic pancreatitis COX-2 overexpression was also recently observed[44]. The enzyme was found in atrophic acinar cells, hyperplastic ductal cells and islets cells. As chronic pancreatitis is a risk factor for pancreatic cancer, this opens whether COX-2 is involved in the tumorigenesis via chronic inflammation. Apart from positive islets cells, we could not see any COX-2 expression in normal tissues, especially in the surrounding tissue of the tumor. If COX-2 is constantly expressed in chronic inflammation of pancreas one would expect enzyme induction also in the inflamed peritumoral region. Other reports mentioning the nonneoplastic tissue also found no COX-2 expression. It seems therefore unlikely that this enzyme is involved in early tumor development via inflammation.
In summary, approximately half of pancreatic adenocarcinomas express iNOS and COX-2. Both enzymes are heterogeneously distributed in the tumors without any correlation to each other nor to MVD. COX-2 is a phenomenon of higher malignant tumors. Both enzymes do not appear to be a key step in the angiogenesis of pancreatic adenocarcinomas.
The excellent technical assistance by K. Petmecki, the help in editing the manuscript by Y. A. Weidemann and the statistical support by H. Christ are gratefully acknowledged.
| 1. | Devesa SS, Blot WJ, Stone BJ, Miller BA, Tarone RE, Fraumeni JF. Recent cancer trends in the United States. J Natl Cancer Inst. 1995;87:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 266] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 2. | Bundesamt S. Statistical year book for the Federal Republic of Germany. Metzler Poeschel Stuttgart. 1996;430-433. |
| 3. | Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, Iacobuzio-Donahue CA, Maitra A, Goggins M, Canto MI. Pancreatic cancer. Curr Probl Cancer. 2002;26:176-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Yeo CJ, Abrams RA, Grochow LB, Sohn TA, Ord SE, Hruban RH, Zahurak ML, Dooley WC, Coleman J, Sauter PK. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg. 1997;225:621-633; discussion 633-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 449] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 5. | Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 851] [Article Influence: 35.5] [Reference Citation Analysis (1)] |
| 6. | Sohn TA. The molecular genetics of pancreatic ductal carcinoma. Minerva Chir. 2002;57:561-574. [PubMed] |
| 7. | Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Hall Pde L, Wilentz RE, de Klerk W, Bornman PP. Premalignant conditions of the pancreas. Pathology. 2002;34:504-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 1995] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 10. | Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4289] [Cited by in RCA: 4197] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 11. | Förstermann U, Kleinert H. Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:351-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 282] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Kröncke KD, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotoxicity versus cytoprotection--how, why, when, and where? Nitric Oxide. 1997;1:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 380] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Ambs S, Ogunfusika MO, Merriam WG, Bennett WP, Billiar TR, Harris CC. Up-regulation of inducible nitric oxide synthase expression in cancer-prone p53 knockout mice. Proc Natl Acad Sci USA. 1998;95:8823-8828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG, Billiar TR, Harris CC. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334-341. [PubMed] |
| 15. | Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908-7916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1061] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 16. | Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157-33160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1341] [Cited by in RCA: 1330] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 17. | Lin DT, Subbaramaiah K, Shah JP, Dannenberg AJ, Boyle JO. Cyclooxygenase-2: a novel molecular target for the prevention and treatment of head and neck cancer. Head Neck. 2002;24:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Kasper HU, Ebert M, Malfertheiner P, Roessner A, Kirkpatrick CJ, Wolf HK. Expression of thrombospondin-1 in pancreatic carcinoma: correlation with microvessel density. Virchows Arch. 2001;438:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue]. Pathologe. 1987;8:138-140. [PubMed] |
| 20. | Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 3201] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 21. | Tarta C, Teixeira CR, Tanaka S, Haruma K, Chiele-Neto C, da Silva VD. Angiogenesis in advanced colorectal adenocarcinoma with special reference to tumoral invasion. Arq Gastroenterol. 2002;39:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Sauer G, Deissler H. Angiogenesis: prognostic and therapeutic implications in gynecologic and breast malignancies. Curr Opin Obstet Gynecol. 2003;15:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C, Sculier JP. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2002;87:694-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Chiarugi V, Magnelli L, Gallo O. Cox-2, iNOS and p53 as play-makers of tumor angiogenesis (review). Int J Mol Med. 1998;2:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Kong G, Kim E, Kim W, Lee Y, Lee J, Paik S, Rhee J, Choi K, Lee K. Inducible nitric oxide synthase (iNOS) immunoreactivity and its relationship to cell proliferation, apoptosis, angiogenesis, clini-copathologic characteristics, and patient survival in pancreatic cancer. Int J Gastrointest Cancer. 2001;29:133-140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Kong G, Kim EK, Kim WS, Lee KT, Lee YW, Lee JK, Paik SW, Rhee JC. Role of cyclooxygenase-2 and inducible nitric oxide synthase in pancreatic cancer. J Gastroenterol Hepatol. 2002;17:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Wolf H, Haeckel C, Roessner A. Inducible nitric oxide synthase expression in human urinary bladder cancer. Virchows Arch. 2000;437:662-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Thomsen LL, Lawton FG, Knowles RG, Beesley JE, Riveros-Moreno V, Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994;54:1352-1354. [PubMed] |
| 29. | De Paepe B, Verstraeten VM, De Potter CR, Bullock GR. Increased angiotensin II type-2 receptor density in hyperplasia, DCIS and invasive carcinoma of the breast is paralleled with increased iNOS expression. Histochem Cell Biol. 2002;117:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Ambs S, Bennett WP, Merriam WG, Ogunfusika MO, Oser SM, Khan MA, Jones RT, Harris CC. Vascular endothelial growth factor and nitric oxide synthase expression in human lung cancer and the relation to p53. Br J Cancer. 1998;78:233-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA. 1995;92:4392-4396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 567] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 32. | Nicotera P, Ankarcrona M, Bonfoco E, Orrenius S, Lipton SA. Neuronal necrosis and apoptosis: two distinct events induced by exposure to glutamate or oxidative stress. Adv Neurol. 1997;72:95-101. [PubMed] |
| 33. | Dimmeler S, Rippmann V, Weiland U, Haendeler J, Zeiher AM. Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ Res. 1997;81:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 230] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Tzeng E, Kim YM, Pitt BR, Lizonova A, Kovesdi I, Billiar TR. Adenoviral transfer of the inducible nitric oxide synthase gene blocks endothelial cell apoptosis. Surgery. 1997;122:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Gansauge S, Nussler AK, Beger HG, Gansauge F. Nitric oxide-induced apoptosis in human pancreatic carcinoma cell lines is associated with a G1-arrest and an increase of the cyclin-dependent kinase inhibitor p21WAF1/CIP1. Cell Growth Differ. 1998;9:611-617. [PubMed] |
| 36. | Ricchi P, Zarrilli R, Di Palma A, Acquaviva AM. Nonsteroidal anti-inflammatory drugs in colorectal cancer: from prevention to therapy. Br J Cancer. 2003;88:803-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1733] [Cited by in RCA: 1682] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 38. | Molina MA, Sitja-Arnau M, Lemoine MG, Frazier ML, Sinicrope FA. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59:4356-4362. [PubMed] |
| 39. | Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS, Sweeney CJ. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 227] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, Kosuge T, Yoshida S. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001;91:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 41. | Okami J, Yamamoto H, Fujiwara Y, Tsujie M, Kondo M, Noura S, Oshima S, Nagano H, Dono K, Umeshita K. Overexpression of cyclooxygenase-2 in carcinoma of the pancreas. Clin Cancer Res. 1999;5:2018-2024. [PubMed] |
| 42. | Koshiba T, Hosotani R, Miyamoto Y, Wada M, Lee JU, Fujimoto K, Tsuji S, Nakajima S, Doi R, Imamura M. Immunohistochemical analysis of cyclooxygenase-2 expression in pancreatic tumors. Int J Pancreatol. 1999;26:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Niijima M, Yamaguchi T, Ishihara T, Hara T, Kato K, Kondo F, Saisho H. Immunohistochemical analysis and in situ hybridization of cyclooxygenase-2 expression in intraductal papillary-mucinous tumors of the pancreas. Cancer. 2002;94:1565-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Schlosser W, Schlosser S, Ramadani M, Gansauge F, Gansauge S, Beger HG. Cyclooxygenase-2 is overexpressed in chronic pancreatitis. Pancreas. 2002;25:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Edited by Wang XL Proofread by Xu FM