Published online Jul 1, 2004. doi: 10.3748/wjg.v10.i13.1914
Revised: May 22, 2003
Accepted: June 2, 2003
Published online: July 1, 2004
AIM: To investigate the effects of traditional Chinese medicinal enema (TCME) on inflammatory and immune response of colonic mucosa of rats with ulcerative colitis (UC), and to observe the pathogenic mechanism.
METHODS: Thirty UC rats, induced by intestinal enema together with 2.4-dinitrochlorobenzene (DNCB) and acetic acid, were randomly divided into 3 groups, i.e., G I, G II and G III. Groups G I and G II were administered with TCME and salazosulfapyridine enema (SASPE), respectively. Group G III was clystered with only normal saline (NSE), served as control. Group G IV was taken from normal rats as reference, once daily, from the 7th day after the establishment of UC for total 28 d. Interleukin-6 (IL-6) in the colonic mucosa was assayed by 3H-TdR incorporation assay. Colonic mucosal lymphocyte subpopulation adhesive molecules, CD4+CD11a+, CD4+CD18+, CD8+CD11a+, CD8+CD18+ (LSAM), tumor necrosis factor (TNF)-α, and interferon-γ (IFN-γ), were detected by enzyme linked immunosorbent assay (ELISA). Moreover, the expression of TNF-α mRNA and IFN-γ mRNA in colonic mucosa were detected by polymerase chain reaction (RT-PCR).
RESULTS: Before therapies, in model groups, G I, G II and G III, levels of IL-6, TNF-α, IFN-γ, CD8+CD11a+ and CD8+CD18+ were significantly different (38.29 ± 2.61 U/mL, 16.54 ± 1.23 ng/L, 8.61 ± 0.89 ng/L, 13.51% ± 2.31% and 12.22% ± 1.13%, respectively) compared to those in G IV group (31.56 ± 2.47 U/mL, 12.81 ± 1.38 ng/L, 5.28 ± 0.56 ng/L, 16.68% ± 1.41% and 16.79% ± 1.11%, respectively). After therapeutic enemas, in G I group, the contents of IL-6 (32.48 ± 2.53 U/m), TNF-α (13.42 ± 1.57 ng/L) and IFN-γ (5.87 ± 0.84 ng/L) were reduced; then, the contents of CD8+CD11a+ (16.01% ± 1.05 %) and CD8+CD18+ (16.28% ± 0.19%) were raised. There was no significant difference between groups G I and G IV, but the difference between groups G I and G II was quite obvious (P < 0.05). The expressions of TNF-α mRNA and IFN-γ mRNA in group G III were much higher than those of group G IV, but those in group G I were significantly suppressed by TCME therapy.
CONCLUSION: Ulcerative colitis is related to colonic regional mucosal inflammatory factors and immune imbalance. TCME can effectively inhibit regional mucosal inflammatory factors and improve their disorder of immunity.
- Citation: Guo SM, Tong HB, Bai LS, Yang W. Effect of traditional Chinese medicinal enemas on ulcerative colitis of rats. World J Gastroenterol 2004; 10(13): 1914-1917
- URL: https://www.wjgnet.com/1007-9327/full/v10/i13/1914.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i13.1914
Ulcerative colitis (UC) is a refractory, chronic and non-specific disease. Its pathogenesis is probably related to the deficiency of autoimmunity and imbalance of immunoregulation[1-5]. The relationship between pathogenesis and immunity of colonic mucosa remains a focus at present[6-11]. In view of poor curative effect and high recurrence rate, traditional Chinese medicinal formulae were used to treat the disease in recent years, and the therapeutic effectiveness is quite satisfactory[12]. In order to explore the mechanism of UC and pharmacological action of the traditional Chinese medicinal formulae, rat models were established to observe the effect of traditional Chinese medicinal enema on immunological and inflammatory factors in colonic mucosa of rats with UC.
Six-week-old Wistar rats (n = 56), half of male and half of female were purchased from Shanghai Laboratory Animal Center of Chinese Academy of sciences (SLAC. CAS), calf serum, ConA and PHA (Institute of Cell Research of CAS) and mRNA reagent kit and PCR reagent (Promega), IL-6, TNF-α, IFN-γ reagent kit and PE marked CD11a+, CD18+ monoclonal antibody (Sigma). 3H-TdR was from Shanghai Atomic Nuclear Institute of CAS. Cell factor primer-detonator was purchased from Shanghai Biological Engineering Center of CAS. The formulae of traditional Chinese medicinal herbs for enema consisted of Huangqi (astragalus), Dahuang (caulis fibraureae), Huangbai (cortex phellodendri), Wubeizi (galla chinensis) and Baiji (rhizoma bletillae), mixed with 1g crude drug per milliliter by Medicament Section of Shanghai Tongji Hospital. Salazosulfapyridine (SASP, batch No.921101, Shanghai Sine Pharmaceutical Factory) was prepared as suspension (20 mg/mL) at the same section.
The models were prepared in reference to literature[13]. Fifty six Wistar rats weighing 200 ± 20 g, were fed for 1 week before experiment. Then 38 rats were randomly taken as subjects for experiment. After depilation on back and neck, the rats were administered 0.3 mL of DNCB acetone liquid (DNCB 2.0 g: acetone 100 mL) solution on their bare backs and necks once daily for total 14 d. At the 15th day, 0.25 mL of 0.04 mmol/L DNCB ethanol (500 mL/L) solution was infused through a nylon hose inserted into the colon of rat 8 cm depth. At the 16th day, 2 mL of 80 mL/L-1 acetic acid solution was infused at the same depth. Then, the colon was rinsed with 5 mL normal saline after 15 s of retention. The remaining 18 rats acted as controls after infusion of normal saline (NS) at 1 week since the preparation of the models, 8 rats were taken respectively from the group of model and group for reference to detect the levels of colonic mucosa lymphocytes: IL-6, TNF-α, IFN-γ and LSAM. The remaining 30 rats were randomly divided into 3 groups of 10 each: G I, G II and G III were clystered daily with 2 mL of TCM, SASP and NS for 28 d respectively. Another group (G IV) of 10 rats, administered 2 mL of NS daily for 28 d, was taken as reference of normality. At the end of 4 wk experimental period, the lymphocytes were again detected and also the expressions of TNF-α mRNA and IFN-γ mRNA were examined.
Separation and culture of intestinal theca mucosa cells Mucosa cells were separated and routinely cultured to prepare monolayer culture cells suspension of colonic mucosa, and the suspension was adjusted to 5 × 109 /L in RPMI 1640 containing 100 mL/L calf serum. Then, 1.0 mL of suspension, was put in 24-well plate, then 1.0 mL of ConA (final density 5 mg/L) was added, and cultured at 37 °C for 24 h in a humidified atmosphere containing 50 mL/L CO2. The supernatant of the culture was centrifuged at 3 000 r/min and preserved at -20 °C for detection of IL-6, TNF-α, IFN-γ and LSAM levels according to the manufacturer’s instructions.
Total RNA from colonic mucosa tissue of rats was extracted as previously described[14-16]: using single-step method of RNA isolation (acid guanidinium thiocyanate-phenol-chloroform extraction). Formaldehyde denatured electrophoresis stained with ethidium bromide (EB) was used to examine infect RNA. Reverse transcription reaction was performed as previously described[17-19]: a total amount of l μg of RNA as template was mixed with RNasin (40 U/μL) 0.5 μL, 25 μmol/L oligo (dT) 1 μL, 5 × RT buffer 5 μL, dNTP (10 mmol/L) 1 μL and AMV (9 U/μL) 1 μL to a total reaction volume of 25 μL, followed by incubation at 37 °C for 1 h and at 94 °C for 5 min. PCR reaction was carried out as described previously[20-22]: 10 μL cDNA was added with 0.1 μg of primer, 0.05 μg of β-action primer and 2.5 U of Taq enzyme (5 U/μL). Total PCR reaction volume was 100 μL. PCR condition was as follows: deraturation at 94 °C for 1 min; primer annealing at 54 °C for 40 s, and extension at 72 °C for 40 s, followed a further extension at 72 °Cfor 7 min. Other cycles were as follows: after 30 cycles of amplification, 20 μL of PCR product was electrophoresed on 20 g/L agarose gel, then recorded with camera under ultraviolet for density scanning and calculated the relative expression of the gene.
Experimental results were expressed as mean ± SD. P < 0.05 was considered statistically significant. All statistical calculations were performed using the SPSS for Windows version 9.0 software package.
At 1 week after setting of models, the expression of cytokines in MG was found to be significantly higher than that in CG (P < 0.01, Table 1). The expressions of lymphocyte T subpopulation surface adhesive molecules, CD8+CD11a+ and CD8+CD18+, were remarkably lower in MG than those in CG (P < 0.01, Table 2).
In group G I clystered with TCME, the levels of IL-6, TNF-αand IFN-γ were obviously reduced. Levels of CD8+ CD11a+ and CD8+ CD18+ were remarkably raised, which were no obvious differences compared with those in G IV group (i.e., control group) (P > 0.05, Table 3 and Table 4), but, there were significant differences between groups G I and G II (P < 0.05, Table 3 and Table 4).
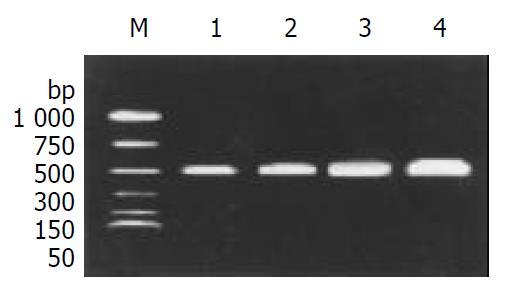
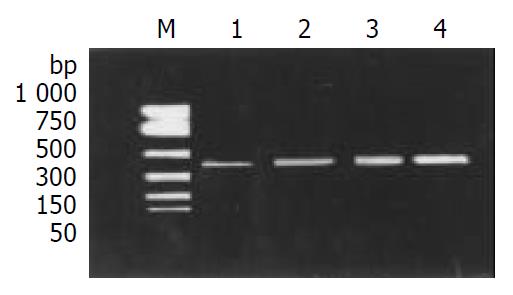
The expression of TNF-α mRNA and IFN-γ mRNA in colonic mucosal tissue, was higher in G III group than those in G IV group (Figure 1, Figure 2). After treatment, TNF-α mRNA and IFN-γ mRNA levels were obviously suppressed in groups G I and G II, especially in group G I.
The pathogenesis of colonic colitis remains unclear up to now. It is believed that colonic colitis results from the interaction of many factors, such as environment, immunity and heredity, etc. Probably, susceptible population in heredity is affected by environmental factors, such as water, food and infection, which trigger excessive reaction of intestinal immunity. The reaction can cause an inflammatory stimulation to intestinal mucosa and damage it[24,25]. More and more attention has been paid to autoimmunity. An opinion suggests that there exists an inevitable correlation between the immunity system of intestinal mucosa and its integrity[26-28]. It has been reported that IL-6, a mononuclear macrophage, is a cytokine secreted by T and B cell. IL-6 can promote proliferation of T cell and enhance T cell reaction to cell toxicity. Our experimental result showed that IL-6 was high active in colonic mucosal tissue of UC models, the higher level of IL-6 can further aggravate the injury on colonic tissue by antigen-antibody reaction and complement activation[29-35]. More endogenous IFN-γ and TNF-α produced by colonic epithelia of UC models cause the changes in epithelia, which may lead directly to scathes of inflammatory epithelia[36,37]. Disorder of autoimmunity is closely related to the imbalance of Th1 and Th2 cells. For IFN-γ being on Th1 cell, abnormal expressions of IFN-γ and IFN-γ mRNA cause inevitably the imbalance of Th1/Th2 and result in immune disorder[38-40]. CD11a+/CD18+ are cell surface adhesive albumin, also known as lymphocyte function antigen-l (LFA-l), a representative of β2 integrants. It possesses a series of functions, such as introduction of adhesion, chemotaxis of cell and homing of lymphocytes, etc. It expresses on surfaces of white cells, phagocytes and large granular lymphocytes, acting as a key channel in interaction and information between cells. A few of cellular factors, affected by them, such as IL-6, IFN-γ, TNF-α and endotoxin, can affect their expressions[41-43]. CD8+CD11a+ and CD8+CD18+ (on Ts cell) were found in suffers with moderate and severe ulcerative colitis, and significantly lower than those in normal control rats[44]. This is in conformity to our findings.
Our study showed that expressions of IL-6, TNF-α, TNF-α mRNA, IFN-γ, IFN-γ mRNA were found to be obviously higher and the levels of lymphocyte T surface adhesive molecules, CD8+CD11a+ and CD8+CD18+ were lower than those in normal rats in colonic mucosal tissue of UC models. The results were in agreement with the findings of others[45]. The results indicated that ulcerative colitis was correlated with disfunction and disorder of autoimmunity. The abnormal expressions of local inflammatory factors and adhesive albumin of cell surface in colonic tissue play a critical role in regulation of immunity. The mutual affects of these factors and abnormality of regulation of immune system are regarded as the core of pathogenesis. The imbalance of regulation between pre-inflammation cytokines and anti-inflammation cytokines is considered an important mechanism of intestine inflammation, including ulcerative colitis.
At present, ulcerative colitis lacks more effective therapy for radical cure. Principally, steroid hormones and salicylic acid preparation are used to control and suppress the inflammation. In recent years, along with the development of immunology and molecular biology, the accumulation of knowledge about the disease and further understanding of the mechanism of drug action, new methods of treatment have come up into being one after another. But the ordinary UC sufferers can not afford remedies due to the exorbitant price of drugs, and the safety of the remedies needs to be further identified. Our study demonstrated that salazosulfapyridine (SASP) is still the principal remedy for ulcerative colitis. It can improve the conditions of patients in mild and moderate states. But the side-effect and high relapse rate after the remedy are unsatisfactory. Traditional Chinese medicine is popular in China. It is characteristic of TCM to treat intestinal diseases with TCM herbs enemas[12]. The study showed that the treatment of TCME was better on UC than SASPE. Our TCME could effectively inhibit the activity of granulocyte, macrophage and monocyte. Also it could reduce immune response and formation of inflammation in colonic mucosal tissue, which might be due to that our TCME could reduce the expressions of IL-6, TNF-α, IFN-γ and raise the levels of surface adhesive molecules (CD8+CD11a+ and CD8+CD18+), could also suppress the abnormal expressions of IFN-α and IFN-γ mRNA, improve the disorder of immunity in UC and the Baiji could protect colonic mucosa, Bahuang and Huangbai could promote cruor. SASP suspension has more side-effects than the formulae of TCM herbs, although it has a certain effect in treatment of ulcerative colitis clinically. Therefore, the retention enema prepared from TCM herbs may be an ideal choice to manage the disease.
| 1. | Furrie E, Macfarlane S, Cummings JH, Macfarlane GT. Systemic antibodies towards mucosal bacteria in ulcerative colitis and Crohn's disease differentially activate the innate immune response. Gut. 2004;53:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Ioachim E, Michael M, Katsanos C, Demou A, Tsianos EV. The immunohistochemical expression of metallothionein in inflammatory bowel disease. Correlation with HLA-DR antigen expression, lymphocyte subpopulations and proliferation-associated indices. Histol Histopathol. 2003;18:75-82. [PubMed] |
| 3. | Ludwiczek O, Kaser A, Tilg H. Plasma levels of soluble CD40 ligand are elevated in inflammatory bowel diseases. Int J Colorectal Dis. 2003;18:142-147. [PubMed] |
| 4. | Sawada-Hase N, Kiyohara T, Miyagawa J, Ueyama H, Nishibayashi H, Murayama Y, Kashihara T, Nakahara M, Miyazaki Y, Kanayama S. An increased number of CD40-high monocytes in patients with Crohn's disease. Am J Gastroenterol. 2000;95:1516-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Candelli M, Nista EC, Nestola M, Armuzzi A, Silveri NG, Gasbarrini G, Gasbarrini A. Saccharomyces cerevisiae-associated diarrhea in an immunocompetent patient with ulcerative colitis. J Clin Gastroenterol. 2003;36:39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Ohman L, Franzén L, Rudolph U, Harriman GR, Hultgren Hörnquist E. Immune activation in the intestinal mucosa before the onset of colitis in Galphai2-deficient mice. Scand J Immunol. 2000;52:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Fukushima K, Yonezawa H, Fiocchi C. Inflammatory bowel disease-associated gene expression in intestinal epithelial cells by differential cDNA screening and mRNA display. Inflamm Bowel Dis. 2003;9:290-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Fahlgren A, Hammarström S, Danielsson A, Hammarström ML. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin Exp Immunol. 2003;131:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Carvalho AT, Elia CC, de Souza HS, Elias PR, Pontes EL, Lukashok HP, de Freitas FC, Lapa e Silva JR. Immunohistochemical study of intestinal eosinophils in inflammatory bowel disease. J Clin Gastroenterol. 2003;36:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Carty E, De Brabander M, Feakins RM, Rampton DS. Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut. 2000;46:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | McKaig BC, McWilliams D, Watson SA, Mahida YR. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol. 2003;162:1355-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Jiang XL, Cui HF. An analysis of 10218 ulcerative colitis cases in China. World J Gastroenterol. 2002;8:158-161. [PubMed] |
| 13. | Padol I, Huang JQ, Hogaboam CM, Hunt RH. Therapeutic effects of the endothelin receptor antagonist Ro 48-5695 in the TNBS/DNBS rat model of colitis. Eur J Gastroenterol Hepatol. 2000;12:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Tahara K, Fujii K, Yamaguchi K, Suematsu T, Shiraishi N, Kitano S. Increased expression of P-cadherin mRNA in the mouse peritoneum after carbon dioxide insufflation. Surg Endosc. 2001;15:946-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Xiang X, Qiu D, Hegele RD, Tan WC. Comparison of different methods of total RNA extraction for viral detection in sputum. J Virol Methods. 2001;94:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Yamada H, Koizumi S. Lymphocyte metallothionein-mRNA as a sensitive biomarker of cadmium exposure. Ind Health. 2001;39:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Chambaut-Guérin AM, Klein J, Paschke R. Interleukin-6 is a positive regulator of tumor necrosis factor alpha-induced adipose-related protein in 3T3-L1 adipocytes. FEBS Lett. 2004;560:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Barbier M, Vidal H, Desreumaux P, Dubuquoy L, Bourreille A, Colombel JF, Cherbut C, Galmiche JP. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol Clin Biol. 2003;27:987-991. [PubMed] |
| 19. | Ridyard AE, Nuttall TJ, Else RW, Simpson JW, Miller HR. Evalu-ation of Th1, Th2 and immunosuppressive cytokine mRNA ex-pression within the colonic mucosa of dogs with idiopathic lym-phocytic-plasmacytic colitis. Vet Immunol Immunopathol. 2002;86:205-214. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, McLeod RS, Griffiths AM, Green T, Brettin TS, Stone V, Bull SB. Genomewide search in Canadian families with inflammatory bowel disease reveals two novel susceptibility loci. Am J Hum Genet. 2000;66:1863-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 362] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 21. | Akazawa A, Sakaida I, Higaki S, Kubo Y, Uchida K, Okita K. Increased expression of tumor necrosis factor-alpha messenger RNA in the intestinal mucosa of inflammatory bowel disease, particularly in patients with disease in the inactive phase. J Gastroenterol. 2002;37:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 413] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Sawa Y, Oshitani N, Adachi K, Higuchi K, Matsumoto T, Arakawa T. Comprehensive analysis of intestinal cytokine messenger RNA profile by real-time quantitative polymerase chain reaction in patients with inflammatory bowel disease. Int J Mol Med. 2003;11:175-179. [PubMed] |
| 24. | Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Büchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 311] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 25. | Elson CO, Sartor RB, Targan SR, Sandborn WJ. Challenges in IBD Research: updating the scientific agendas. Inflamm Bowel Dis. 2003;9:137-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, Landers CJ, Abreu-Martin MT, Rotter JI, Yang H. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology. 2004;126:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 372] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 27. | Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, McLeod RS, Griffiths AM, Green T, Brettin TS, Stone V, Bull SB. Genomewide search in Canadian families with inflammatory bowel disease reveals two novel susceptibility loci. Am J Hum Genet. 2000;66:1863-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 362] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 28. | Obermeier F, Schwarz H, Dunger N, Strauch UG, Grunwald N, Schölmerich J, Falk W. OX40/OX40L interaction induces the expression of CXCR5 and contributes to chronic colitis induced by dextran sulfate sodium in mice. Eur J Immunol. 2003;33:3265-3274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Beil WJ, McEuen AR, Schulz M, Wefelmeyer U, Kraml G, Walls AF, Jensen-Jarolim E, Pabst R, Pammer J. Selective alterations in mast cell subsets and eosinophil infiltration in two complementary types of intestinal inflammation: ascariasis and Crohn's disease. Pathobiology 2002-. 2003;70:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Stucchi AF, Shebani KO, Leeman SE, Wang CC, Reed KL, Fruin AB, Gower AC, McClung JP, Andry CD, O'Brien MJ. A neurokinin 1 receptor antagonist reduces an ongoing ileal pouch inflammation and the response to a subsequent inflammatory stimulus. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1259-G1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 210] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 32. | Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597-3603. [PubMed] [DOI] [Full Text] |
| 33. | Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 549] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 34. | Sandborn WJ. Evidence-based treatment algorithm for mild to moderate Crohn's disease. Am J Gastroenterol. 2003;98:S1-S5. [PubMed] |
| 35. | Gasche C, Bakos S, Dejaco C, Tillinger W, Zakeri S, Reinisch W. IL-10 secretion and sensitivity in normal human intestine and inflammatory bowel disease. J Clin Immunol. 2000;20:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Abreu MT, Taylor KD, Lin YC, Hang T, Gaiennie J, Landers CJ, Vasiliauskas EA, Kam LY, Rojany M, Papadakis KA. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease. Gastroenterology. 2002;123:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 331] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Suryaprasad AG, Prindiville T. The biology of TNF blockade. Autoimmun Rev. 2003;2:346-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Loher F, Bauer C, Landauer N, Schmall K, Siegmund B, Lehr HA, Dauer M, Schoenharting M, Endres S, Eigler A. The interleukin-1 beta-converting enzyme inhibitor pralnacasan reduces dextran sulfate sodium-induced murine colitis and T helper 1 T-cell activation. J Pharmacol Exp Ther. 2004;308:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Topilski I, Harmelin A, Flavell RA, Levo Y, Shachar I. Preferential Th1 immune response in invariant chain-deficient mice. J Immunol. 2002;168:1610-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 212] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Noti JD, Johnson AK, Dillon JD. Structural and functional characterization of the leukocyte integrin gene CD11d. Essential role of Sp1 and Sp3. J Biol Chem. 2000;275:8959-8969. [PubMed] |
| 42. | Schneeberger EE, Vu Q, LeBlanc BW, Doerschuk CM. The accumulation of dendritic cells in the lung is impaired in CD18-/- but not in ICAM-1-/- mutant mice. J Immunol. 2000;164:2472-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Tilg H, Vogelsang H, Ludwiczek O, Lochs H, Kaser A, Colombel JF, Ulmer H, Rutgeerts P, Krüger S, Cortot A. A randomised placebo controlled trial of pegylated interferon alpha in active ulcerative colitis. Gut. 2003;52:1728-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Vainer B, Brimnes J, Claesson MH, Nielsen OH. Impaired sensitivity to beta 2 integrin-blocking in ICAM-1-mediated neutrophil migration in ulcerative colitis. Scand J Gastroenterol. 2001;36:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Vainer B, Sørensen S, Seidelin J, Nielsen OH, Horn T. Expression of ICAM-1 in colon epithelial cells: an ultrastructural study performed on in vivo and in vitro models. Virchows Arch. 2003;443:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Edited by Kumar M and Chen WW Proofread by Xu FM