Published online Jun 1, 2004. doi: 10.3748/wjg.v10.i11.1612
Revised: December 4, 2003
Accepted: December 8, 2003
Published online: June 1, 2004
AIM: To investigate the modification of baculovirus vector and the feasibility of delivering exogenous genes into mammalian cells with the culture supernatant of Spodoptera frugiperta (Sf9) cells infected by recombinant baculoviruses.
METHODS: Two recombinant baculoviruses (BacV-CMV-EGFPA, BacV-CMV-EGFPB) containing CMV-EGFP expression cassette were constructed. HepG2 cells were directly incubated with the culture supernatant of Sf9 cells infected by recombinant baculoviruses, and reporter gene transfer and expression efficiencies were analyzed by flow cytometry (FCM). The optimal transduction conditions were investigated by FCM assay in HepG2 cells. Gene-transfer and expression efficiencies in HepG2 or CV1 cells by baculovirus vectors were compared with lipofectAMINE, recombinant retrovirus and vaccinia virus expression systems. Twenty different mammalian cell lines were used to investigate the feasibility of delivering exogenous genes into different mammalian cells with the culture supernatant of infected Sf9 cells.
RESULTS: CMV promoter could directly express reporter genes in Sf9 cells with a relatively low efficiency. Target cells incubated with the 1:1 diluted culture supernatant (moi = 50) for 12 h at 37 °C could achieve the highest transduction and expression efficiencies with least impairment to cell viability. Under similar conditions the baculovirus vector could achieve the highest gene-transfer and expression efficiency than lipofectAMINE, recombinant retrovirus and vaccinia virus expression systems. Most mammalian cell lines could be transduced with recombinant baculovirus. In primate adherent culture cells the recombinant baculovirus could arrive the highest infection and expression efficiencies, but it was not very satisfactory in the cell lines from mice and suspended culture cells.
CONCLUSION: Mammalian cells incubated with the culture supernatant of infected Sf9 cells could serve as a very convenient way for rapid and efficient expression of foreign genes in mammalian cells, but it might be more suitable for primate adherent culture cells.
- Citation: Cheng T, Xu CY, Wang YB, Chen M, Wu T, Zhang J, Xia NS. A rapid and efficient method to express target genes in mammalian cells by baculovirus. World J Gastroenterol 2004; 10(11): 1612-1618
- URL: https://www.wjgnet.com/1007-9327/full/v10/i11/1612.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i11.1612
The baculovirus (Autographa californica multiple nuclear polyhedrosis virus, AcMNPV) insect cell expression system has been extensively developed and widely used for the production of numerous recombinant proteins in insect cells[1-5]. As the previous reports described, baculovirus had a strict host range, which was only limited to lepidopteran insects. However, researchers have reported that baculoviruses can be taken up by some mammalian cells[6,7], but are incapable of replicating in these mammalian cells[8,9]. A modified AcMNPV containing promoters that are active in mammalian cells, such as Rous sarcoma virus (RSV) promoter and cytomegalovirus immediate early (CMV-IE) promoter, can express exogenous genes in mammalian cells[10-14]. So a new way could be chosen by researchers for experiments of delivering target genes into mammalian cells, besides the conventional lipid transfection and mammalian viral vector expression systems, such as retrovirus expression system, adenovirus expression system. Previous reports have described that recombinant baculoviruses used in gene-transfer experiments were often concentrated by ultracentrifugation. Although this way can markedly increase the virus titer, but it needs to culture a large number of cells to obtain sufficient viruses, and the manipulation is complex and burdensome. So it is inconvenient in some daily common experiments.
Bac-to-Bac system is the most often used baculovirus-based expression system for the production of recombinant proteins in insect cells. In our research, based on the Bac-to-Bac system recombinant baculoviruses were constructed, which contain the enhanced green fluorescent protein (eGFP) gene driven by CMV promoter, to investigate the modification of baculovirus vector and the feasibility of delivering exogenous genes into mammalian cells with the culture supernatant of Sf9 cells infected by recombinant baculoviruses. Compared with lipid transfection, retrovirus and vaccinia virus expression system, efficiencies of gene transfer and expression in mammalian cells by the culture supernatant of infected Sf9 cells were superior to the traditional ways. Since direct application of the culture supernatant could simplify the procedures of delivering foreign genes into mammalian cells by baculovirus vectors, it could serve as a valuable tool for some daily common experiments.
Spodoptera frugiperda (Sf9) cell line was purchased from Invitrogen (California, USA). CV1, 293, 143B, HepG2, PLC/PRF/5, BNL 1ME A.7R.1, WI-38, DMS-114, JC, L-929, P815, PT67 cell lines were obtained from the American Type Culture Collection. Hela, CHO, NIH3T3, Raji, CNE, MCF-7, BGC-223 cell lines were stored in our laboratory. LCL-cm and pT67-EGFP cell lines were constructed in our laboratory.
E.coli DH5a was stored in our laboratory. E.coli DH10Bac was purchased from Invitrogen (California, USA). pcDNA3.1 (+) was purchased from Invitrogen (California, USA). pEGFP was purchased from Clontech (California, USA). pMD18-EF1A, pCDNA3.1-EGFP was constructed in our laboratory.
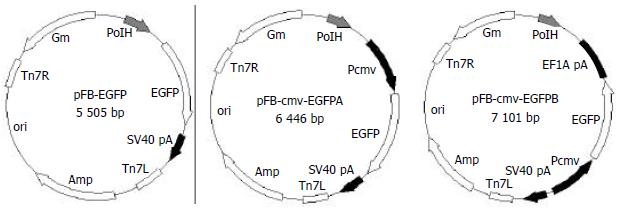
Plasmid pEGFP was digested with BamHI and EcoRI, a 760 bp fragment containing EGFP gene was retrieved and inserted into the pFastBacl backbone that was digested with BamHI and EcoRI to form pFB-EGFP. EGFP gene was moved from pEGFP to pcDNA3.1 (+) as an EcoRI-BamHI fragment to construct pN31-EGFP. An 1.6 kb BglII-EcoRI fragment from pN31-EGFP, containing CMV-IE promoter/enhancer and EGFP gene, was inserted into the pFastBacl backbone which was digested with BamHI and EcoRI to obtain pFB-CMV-EGFPA. A BglII-BamHI fragment containing the polyadenylation signal was inserted into the BamHI site of pFastBacl to construct pFB-EF1A. An 1.6 kb SalI-EcoRI fragment from pN31-EGFP was inserted into pFB-EF1A which was digested with XhoI and EcoRI to construct pFB-CMV-EGFPB (Figure 1).
Shuttle vectors pFB-EGFP, pFB-CMV-EGFPA, pFB-CMV-EGFPB were transformed into E.coli DH10Bac cells, which were incubated on LB agar plates containing 100 µg/mL Bluo-gal, 40 µg/mL IPTG, 7 µg/mL gentamicin, 50 µg/mL kanamycin, 10 µg/mL tetracycline for 24-48 h at 37 °C White colonies were inoculated into LB medium containing the same antibiotics and bacmid DNA was isolated according to the standard manual (Invitrogen).
pUC/M13 amplification primers were directed at sequences on either side of the miniattTn7 site within the lacZa-complementation region of bacmid. If transposition occurred, the PCR product produced by these primers (at 94 °C for 50 s, at 55 °C for 50 s, at 72 °C for 5 min, 30 cycles, at 72 °C for 10 min) was 2300 bp plus the size of the insert. The PCR product of bacmid alone was about 300 bp, bacmid transposed with pFB-EGFP was 3060 bp, bacmid transposed with pFB-CMV-EGFPA was 3978 bp, and bacmid transposed with pFB-CMV-EGFPB was 4633 bp.
Sf9 cells were cultured in Grace’s supplemented insect medium containing 100 mL/L fetal bovine serum (HyClone). Recombinant baculoviruses were generated by Bac-to-Bac system according to the standard manual (Invitrogen). Viruses were amplified to a high titer by propagation in Sf9 cells. Virus titers were measured by plaque assay on Sf9 cells.
Mammalian cells were seeded in 24-well culture dishes about 50000 cells per well and incubated in a 37 °C CO2 incubator for 12 h. Culture medium was removed, replaced with the collected culture supernatant (500 µL), and incubated for 1-24 h at 37 °C After removal of viruses, fresh medium was added and cultures were incubated at 37 °C. Cells that grew in suspension were pelleted by centrifugation before addition of virus inoculums. Forty-eight h post transduction, cultures were examined for eGFP expression using fluorescence microscopy and FCM.
The cells transduced with recombinant baculoviruses were observed by Nikon ECLIPSE TE200 inverted microscope, and fluorescence photos were collected by the digital camera Nikon coolpix990.
After 48 h, transduction cultures were harvested with trypsin and washed with Dulbecco’s PBS. Dispersive cells were pelleted by centrifugation (1500 r/min, 5 min) and resuspended in Dulbecco’s PBS with 50 mL/L fetal bovine serum and filtered by a nylon filter. Data collection was performed on a flow cytometer (FCM, Beckman Coulter EPICS XL), the exciting spectrum was 488 nm, and the detection spectrum was 525 nm. About 20000 signals were collected per specimen. The negative control was the cells without treatment with the viruses, the eGFP positive domain (B domain) was set and the percentage of cell numbers in B domain of the negative control sample did not exceed 2%. The transfer efficiency of reporter genes was obtained by subtracting the percentage of cell numbers in B domain of the negative control from the percentage of cell numbers in B domain of the target sample. The reporter gene expression efficiency was reflected by mean fluorescence intensity of positive cells in the B domain.
Cells were seeded in 24-well culture dishes about 50000 cells per well and incubated in a 37 °C CO2 incubator for 12 h. One µg of target DNA was diluted into 50 µL free-serum culture medium (solution A), 2 µL LipofectAMINE reagent was diluted into 50 µL free-serum culture medium (solution B), the two solutions were mixed gently and incubated for 45 min at room temperature. The cells were washed twice with 1 mL free-serum culture medium. Five hundred µL free-serum culture medium and transfection mixture was added, cells were incubated for 6 h in a 37 °C incubator. The transfection mixture was removed and 500 µL supplemented culture media containing 100 mL/L fetal bovine serum was added. After 48 h transfection cultures were examined for GFP expression by FCM.
pT67-EGFP cell line constructed in our laboratory could produce recombinant retroviruses containing the EGFP expression cassette. Mammalian cells were seeded in 24-well culture dishes about 50000 cells per well and incubated in a 37 °C CO2 incubator for 12 h. The culture medium was removed and replaced with the collected culture supernatant of pT67-EGFP (500 µL), and incubated at 37 °C for 12 h. After removal of the viruses, fresh medium was added and cultures were incubated at 37 °C. After 48 h, infection cultures were examined for GFP expression by FCM.
The recombinant vaccinia viruses containing the EGFP expression cassette were constructed in our laboratory. Mammalian cells were seeded in 24-well culture dishes about 50000 cells per well and incubated in a 37 °C CO2 incubator for 12 h. The culture medium was removed and replaced with the collected vaccinia viruses diluted by PBS (500 µL), and incubated at 37 °C for 1 h. After removal of the viruses, fresh medium was added and cultures were incubated at 37 °C. After 24 h, infection cultures were examined for GFP expression by FCM.
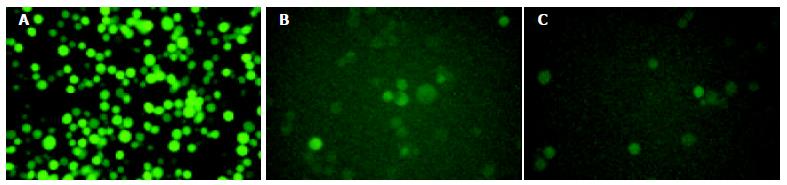
The recombinant baculoviruses (BacV-EGFP, BacV-CMV-EGFPA, BacV-CMV-EGFPB) were constructed with the reporter gene coding for eGFP under the control of either the pHpromoter of baculovirus or the immediate early promoter of CMV. Sf9 cells were infected by the recombinant baculoviruses at a moi of 10. After 72 h, cells were observed by inverted fluorescence microscope (Figure 2). High level of eGFP expression was observed in Sf9 cells infected by BacV-EGFP, whereas low levels of eGFP expression were found in Sf9 cells infected by BacV-CMV-EGFPA or BacV-CMV-EGFPB, showing that CMV promoter was utilized weakly in insect cells, resulting in low expression of eGFP in these cultures.
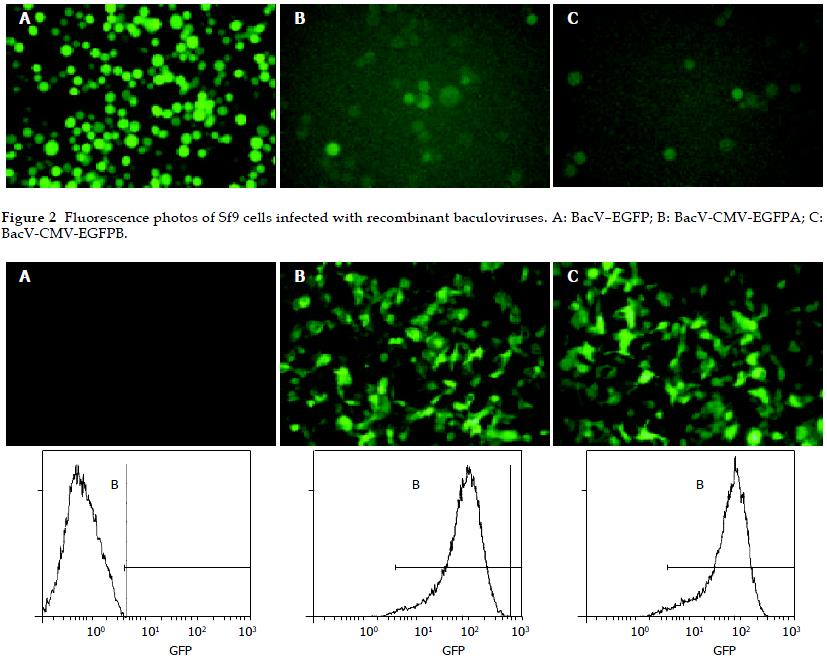
The culture supernatants of Sf9 cells infected by recombinant baculoviruses (BacV-EGFP, BacV-CMV-EGFPA, and BacV-CMV-EGFPB) for 4 d were collected and virus titers were determined by plaque assay. All the collected culture supernatants were diluted with fresh Grace’s culture medium to make the virus titers 1.0 × 107 pfu/mL. HepG2 cells were incubated with the collected supernatants (moi = 100) for 8 h at 37 °C. Twenty-four h post transduction, gene transfer and expression efficiencies were analyzed by inverted microscopy and FCM (Figure 3). High levels of eGFP expression could be detected in HepG2 cells transduced with BacV-CMV-EGFPA or BacV-CMV-EGFPB, and the gene transfer and expression efficiencies were similar. In contrast, no eGFP expression was found in HepG2 cells transduced with BacV-EGFP, showing that the pHpromoter of baculoviruses was inactive in HepG2 cells. During the experiment, the morphological characteristics and growth of HepG2 cells were normal.
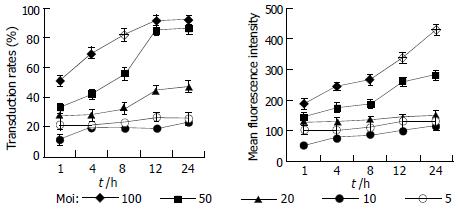
To optimize the way of delivering exogenous genes into mammalian cells with the culture supernatant of Sf9 cells infected by recombinant baculoviruses, HepG2 cells were incubated with the collected culture supernatant serially diluted by DMEM culture medium containing 100 mL/L fetal bovine serum and the transduction time ranged from 1 h to 24 h. After 24 h, the reporter gene transfer and expression efficiencies were analyzed by FCM (Figure 4). With increase of incubation time and moi, the efficiencies of gene transfer and expression in target cells increased.
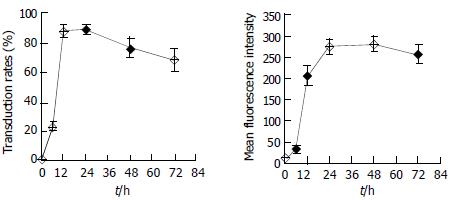
HepG2 cells seeded in 24-well culture dishes were incubated with the culture supernatant of Sf9 cells infected by BacV-CMV-EGFPA (moi = 50). Culture medium was changed every two days. The cells were harvested at various times and quantitatively assayed for eGFP expression by FCM. As shown in Figure 5, the expression of EGFP could be detected during a long time post transduction and peaked 24-48 h after transduction, which implied that the target gene could be continuously expressed in mammalian cells by recombinant baculovirus vectors.
pcDNA3.1 (+) -EGFP was transfected into HepG2 and CV1 cells by LipofectAMINE. After 48 h, the reporter gene transfer and expression efficiencies were analyzed by FCM. pT67-EGFP cell line was constructed in our laboratory, which could produce the recombinant retroviruses containing the EGFP expression cassette. HepG2 and CV1 cells were incubated with the collected culture supernatant of pT67-EGFP for 12 h at 37 °C. After 48 h, the reporter gene transfer and expression efficiencies were analyzed by FCM. HepG2 and CV1 cells were incubated with the culture supernatant of Sf9 cells infected by BacV-CMV-EGFPA for 12 h at 37 °C (moi = 50). After 48 h, the reporter gene transfer and expression efficiencies were analyzed by FCM. The recombinant vaccinia viruses containing the EGFP expression cassette were constructed in our laboratory. HepG2 and CV1 cells were incubated with the collected vaccinia viruses diluted by PBS at 37 °C for 1 h. After 24 h, infection cultures were examined for GFP expression by FCM.
As shown in Table 1, under the similar conditions recombinant baculoviruses and vaccinia viruses could achieve higher gene-transfer and expression efficiencies than lipofectAMINE and recombinant retrovirus system. But obvious cytopathic effects could be observed at 24-32 h on HepG2 or CV1 cells infected by vaccinia viruses, while in mammalian cells transduced with recombinant baculoviruses, no cytopathic effect could be observed during the experiment.
| Gene-transfer rate (%) | Mean fluorescence intensity | |||
| HepG2 | CV1 | HepG2 | CV1 | |
| LipofectAMINE | 29.6 | 34.2 | 138.1 | 103.4 |
| Retro-EGFP | 13.5 | 18.2 | 95.8 | 76.6 |
| Vaccinia-EGFP | 93.5a | 94.2a | 172.5a | 148.41 |
| BacV-CMV-EGFPA | 92.5 | 95.6 | 294.5 | 232.1 |
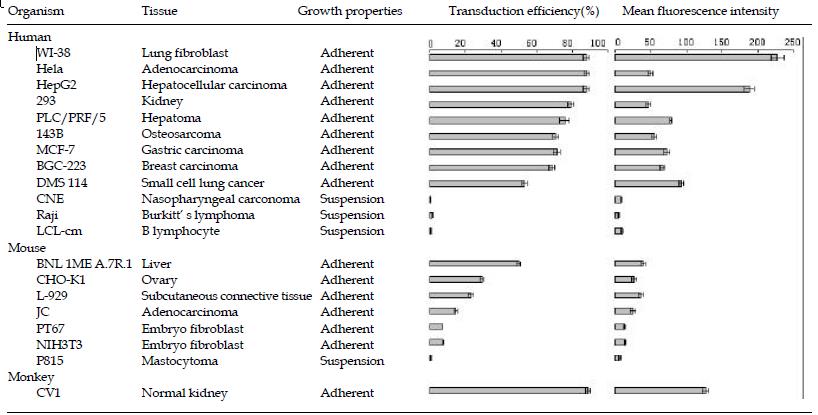
To investigate the feasibility of delivering the reporter gene into various mammalian cells with the culture supernatant of infected Sf9 cells, twenty different mammalian cell lines were used, including twelve human cell lines (WI-38, Hela, HepG2, 293, PLC/PRF/5, 143B, MCF-7, BGC-223, DMS 114, CNE, Raji, LCL-cm), seven mice cell lines (BNL 1ME A.7R.1, CHO-K1, L-929, JC, PT67, NIH3T3, P815), and one monkey cell line (CV1). These cells were incubated with the infected Sf9 cell culture supernatant diluted with complete culture medium for the corresponding mammalian cells at ratio 1:1 (vol:vol) for 12 h (BacV-CMV-EGFPA, moi = 50). After 48 h, the reporter gene transfer and expression efficiencies were analyzed by FCM (Figure 6). Results showed that most mammalian cell lines could be transduced with recombinant baculoviruses by this way.
In our research the susceptibility of mammalian cells lines to recombinant baculoviruses was determined by the expression of reporter gene in different cells. So the results might be affected by different expression efficiencies of reporter gene in different cell lines. The reporter gene used in these experiments was the EGFP gene, which was one of the most widely used reporter genes and had many advantages such as no cytoxicity to host cells, easy detectable, more sensitive[16,17]. The CMV promoter was usually used in experiments, which had the ability to give strong expression of target genes in a variety of mammalian cell types[18].
We investigated the activity of CMV promoter in different mammalian cell lines used in our experiments by FCM. Plasmid pcDNA3.1-EGFP containing an expression cassette of EGFP reporter gene under control of CMV promoter, was transfected by LipofectAMINE into some mammalian cells especially those difficult for baculoviruses to enter. After 48 h, reporter gene transfer and expression efficiencies were analyzed by FCM (Table 2). Results showed that CMV promoter could effectively direct the expression of reporter gene in these mammalian cells. Although the expression efficiencies differed in various cell lines, all the expressions could be detected by FCM. So the gene transfer efficiencies to mammalian cells by recombinant baculoviruses containing CMV-EGFP expression cassette could basically show the ability of baculoviruses to enter different mammalian cell lines.
| Organism | Tissue | Growth properties | Transfection efficiency (%) | Mean fluorescence intensity | |
| Human | |||||
| WI-38 | Lung fibroblast | Adherent | 24.53 | 58.3 | |
| HepG2 | Hepatocellular carcinoma | Adherent | 18.60 | 79.4 | |
| Hela | Adenocarcinoma | Adherent | 19.82 | 42.7 | |
| 293 | Kidney | Adherent | 61.45 | 86.6 | |
| Raji | Burkitt’s lymphoma | Suspension | 5.56 | 14.6 | |
| LCL-cm | B lymphocyte | Suspension | 7.62 | 17.4 | |
| Mouse | |||||
| BNL 1ME A.7R.1 | Liver | Adherent | 13.09 | 19.1 | |
| CHO-K1 | Ovary | Adherent | 47.36 | 48.6 | |
| JC | Adenocarcinoma | Adherent | 12.54 | 22.8 | |
| PT67 | Embryo fibroblast | Adherent | 22.63 | 31.6 | |
| NIH3T3 | Embryo fibroblast | Adherent | 25.31 | 35.3 | |
| P815 | Mastocytoma | Suspension | 12.78 | 16.8 | |
The cells used in our experiments were derived from primate or mice. We compared the transfer efficiencies of reporter gene in adherent or suspended culture cells of these two cell types (Table 3). The result showed that in primate cell lines the efficiencies of gene transfer into adherent culture cells were markedly higher than those of gene transfer into the suspended culture cells (t = 20.6484, P < 0.05). In mice cell lines, only one suspended culture cell line was used in our experiment, its gene transfer efficiency by baculovirus vector was also markedly lower than the adherent culture cell line from the same organism. Furthermore we also noticed that, the efficiencies of gene transfer into the primate adherent culture cells were markedly higher than those of gene transfer into the adherent culture cells from mice (t = 7.9674, P < 0.05), and the efficiencies of reporter gene transfer to the suspended culture cells from primate and mice were both low, the difference between them was not distinct. In primate adherent culture cells recombinant baculoviruses could arrive the highest infection and expression efficiencies, but they were not very satisfactory in the cell lines from mice and suspended culture cells.
| Adherent culture | Suspended culture | ||
| Primate | Total | 10 | 3 |
| Mean transduction efficiency (%) | 77.25 ± 11.37 | 1.12 ± 0.53 | |
| Mice | Total | 6 | 1 |
| Mean transduction efficiency (%) | 21.84 ± 15.85 | 0.61 |
It has been proved that recombinant baculoviruses could serve as a powerful tool for delivering foreign genes into mammalian cells[19]. Previous reports have described that recombinant baculoviruses used in gene-transfer experiments were often concentrated by ultracentrifugation. This could yield a large number of recombinant viruses with high purity, but it needed to culture a large number of cells to obtain sufficient viruses, and the manipulation was complex and burdensome. We investigated the feasibility of delivering exogenous genes into mammalian cells directly with the culture supernatant of Sf9 cells infected by recombinant baculoviruses. The results showed that when the incubation time was identical, with the increase of dilution ratio and the decrease of moi, the reporter gene transfer and expression efficiencies were decreased; when the dilution ratio and moi were identical, with the prolongation of incubation time, the gene transfer and expression efficiencies were increased too, suggesting that virus titers and incubation time were the most important factors that affected the efficiencies of the gene transfer and expression, and the virus titer was the crucial factor. In direct morphological observation in the transduced cells, we found that the growth of target cells was affected when undiluted culture supernatant was used or the incubation time was long. The morphological characteristics of some cells were abnormal, and the number of dead cells increased. So according to the observed results of the morphology and growth of transduced cells, we thought that incubating target mammalian cells with the culture supernatant of infected Sf9 cells (≥ 1.0 × 107pfu/mL) 1:1 (vol:vol) diluted by the mammalian cell complete culture medium for 12 h in a 37 °C CO2 incubator (moi = 50) could achieve the highest efficiency of gene transfer and expression, together with the least impairment to cell viability.
In our research twenty mammalian cell lines were used to investigate the feasibility of delivering reporter genes into various mammalian cells with the culture supernatant of infected Sf9 cells. The results showed that the reporter gene could be effectively transferred into the majority of mammalian cell lines by recombinant baculovirus vectors. The gene transfer efficiencies in adherent culture cells from human or monkey by baculovirus vectors were markedly higher than those from mice, indicating that the susceptibility of mammalian adherent culture cell lines to baculoviruses might be different between different species. Furthermore, baculoviruses could be hardly taken up by suspended culture cells. A total of four suspended culture cell lines were used in our experiment, three from human, and one from mice. Their efficiencies of gene transfer by baculovirus vectors were not more than 2%, markedly lower than those of the adherent culture cell lines from the same species respectively. Similar results were also observed by Condreay et al[20].
Numerous methods have been developed for introducing target genes into mammalian cells including chemical-based procedures, electroporation, and mammalian viral vector-based systems. Lipid transfection, retrovirus expression system and vaccinia virus expression system were most often used in experiments among these methods. The advantages of lipid transfection were short time-used, wide host range, but in common cases the transfection efficiencies were low, and was not suitable for some experiments that needed high transfection efficiencies[21]. Retroviruses could infect many mammalian cell lines, and were able to integrate with host cell genomes stably. But retroviruses could only be taken up by the cells in mitosis phase, and the infection efficiencies in the non dividing cells were very low[22,23]. The successful application of the retrovirus expression system needs high virus titers. If the culture supernatants of retrovirus package cell line were directly used in the gene transfer experiment, the virus titers were usually difficult to meet our need. So increasing the virus titer of recombinant retroviruses has become an important research content in the application of retrovirus expression system, and this was also an important factor that restricted the wide application of the retrovirus expression system[24,25]. A package cell line that can stably generate high titer recombinant viruses would also take a long time for cloning. The vaccinia virus expression systems have been widely used for in vitro production and functional characterization of proteins and live vaccines in vaccine research[26,27]. The life cycle of poxviruses occurs exclusively within the cytoplasm of infected cells and can lead to the lysis of infected cells within 12-24 h[28]. So the vaccinia virus expression system is unsuitable for continuous expression of foreign genes in target cells. Similar problems also occur in application of other mammalian viral vector-based systems. The cost and time-used in application of these expression systems were too expensive and burdensome for some daily common experiments.
As described in our report, the culture supernatant of Sf9 cells infected by recombinant baculoviruses could be directly used for delivery of foreign genes into mammalian cells. Their virus titer was sufficient for efficient gene transfer experiments. So under common circumstances without special requirements, there is no need for concentration and purification of the virus, which can markedly decrease our workloads, and improve our work. In comparison to lipid transfection system, retrovirus expression system and vaccinia virus expression system, we could find that under similar conditions recombinant baculoviruses could achieve the highest gene-transfer and expression efficiencies in mammalian cells. Baculoviruses are inherently unable to replicate in mammalian cells, have few or no microscopically observable cytopathic effects on target cells. These modified recombinant baculoviruses containing mammalian cell-active expression cassettes could be used more widely in a variety of gene transfer. We also noticed that the gene transfer and expression efficiencies in mice cells and suspended culture cells by recombinant baculoviruses containing CMV promoter were not satisfactory, suggesting that although recombinant baculoviruses could serve as a very convenient tool for gene transfer in mammalian cells, but they also have limitations and might not be suitable for all cell lines.
| 1. | Miller LK. Baculoviruses for foreign gene expression in insect cells. Biotechnology. 1988;10:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Luckow VA, Summers MD. Signals important for high-level expression of foreign genes in Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1988;167:56-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 135] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Ren H, Zhu FL, Zhu SY, Song Y, Qi ZT. Immunogenicity of HGV NS5 protein expressed from Sf9 insect cells. World J Gastroenterol. 2001;7:98-101. [PubMed] |
| 4. | Li B, Wu HY, Qian XP, Li Y, Chen WF. Expression, purification and serological analysis of hepatocellular carcinoma associated antigen HCA587 in insect cells. World J Gastroenterol. 2003;9:678-682. [PubMed] |
| 5. | Hou LH, Du GX, Guan RB, Tong YG, Wang HT. In vitro assay for HCV serine proteinase expressed in insect cells. World J Gastroenterol. 2003;9:1629-1632. [PubMed] |
| 6. | Gröner A, Granados RR, Burand JP. Interaction of Autographa californica nuclear polyhedrosis virus with two nonpermissive cell lines. Intervirology. 1984;21:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Carbonell LF, Miller LK. Baculovirus interaction with nontarget organisms: a virus-borne reporter gene is not expressed in two mammalian cell lines. Appl Environ Microbiol. 1987;53:1412-1417. [PubMed] |
| 8. | Tjia ST, zu Altenschildesche GM, Doerfler W. Autographa californica nuclear polyhedrosis virus (AcNPV) DNA does not persist in mass cultures of mammalian cells. Virology. 1983;125:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Carbonell LF, Klowden MJ, Miller LK. Baculovirus-mediated expression of bacterial genes in dipteran and mammalian cells. J Virol. 1985;56:153-160. [PubMed] |
| 10. | Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci USA. 1995;92:10099-10103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 365] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Boyce FM, Bucher NL. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci USA. 1996;93:2348-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 351] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Shoji I, Aizaki H, Tani H, Ishii K, Chiba T, Saito I, Miyamura T, Matsuura Y. Efficient gene transfer into various mammalian cells, including non-hepatic cells, by baculovirus vectors. J Gen Virol. 1997;78:2657-2664. [PubMed] |
| 13. | Delaney WE, Isom HC. Hepatitis B virus replication in human HepG2 cells mediated by hepatitis B virus recombinant baculovirus. Hepatology. 1998;28:1134-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 3.9] [Reference Citation Analysis (2)] |
| 14. | Duisit G, Saleun S, Douthe S, Barsoum J, Chadeuf G, Moullier P. Baculovirus vector requires electrostatic interactions including heparan sulfate for efficient gene transfer in mammalian cells. J Gene Med. 1999;1:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Bac-to-Bac baculovirus expression systems instruction manual. Invitrogen life technologies 2002. . |
| 16. | Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4830] [Cited by in RCA: 4397] [Article Influence: 137.4] [Reference Citation Analysis (3)] |
| 17. | Kain SR, Adams M, Kondepudi A, Yang TT, Ward WW, Kitts P. Green fluorescent protein as a reporter of gene expression and protein localization. Biotechniques. 1995;19:650-655. [PubMed] |
| 18. | Davis MG, Huang ES. Transfer and expression of plasmids containing human cytomegalovirus immediate-early gene 1 promoter-enhancer sequences in eukaryotic and prokaryotic cells. Biotechnol Appl Biochem. 1988;10:6-12. [PubMed] |
| 19. | Kost TA, Condreay JP. Recombinant baculoviruses as mammalian cell gene-delivery vectors. Trends Biotechnol. 2002;20:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 222] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Condreay JP, Witherspoon SM, Clay WC, Kost TA. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci USA. 1999;96:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 338] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Bebök Z, Abai AM, Dong JY, King SA, Kirk KL, Berta G, Hughes BW, Kraft AS, Burgess SW, Shaw W. Efficiency of plasmid delivery and expression after lipid-mediated gene transfer to human cells in vitro. J Pharmacol Exp Ther. 1996;279:1462-1469. [PubMed] |
| 22. | Chuck AS, Clarke MF, Palsson BO. Retroviral infection is limited by Brownian motion. Hum Gene Ther. 1996;7:1527-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Miller DG, Adam MA, Miller AD. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239-4242. [PubMed] |
| 24. | Chuck AS, Palsson BO. Consistent and high rates of gene transfer can be obtained using flow-through transduction over a wide range of retroviral titers. Hum Gene Ther. 1996;7:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Bowles NE, Eisensmith RC, Mohuiddin R, Pyron M, Woo SL. A simple and efficient method for the concentration and purification of recombinant retrovirus for increased hepatocyte transduction in vivo. Hum Gene Ther. 1996;7:1735-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Mackett M, Smith GL, Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. 1982. Biotechnology. 1992;24:495-499. [PubMed] |
| 27. | Whitman ED, Tsung K, Paxson J, Norton JA. In vitro and in vivo kinetics of recombinant vaccinia virus cancer-gene therapy. Surgery. 1994;116:183-188. [PubMed] |
| 28. | Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci USA. 1996;93:11341-11348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 350] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
Edited by Wang XL and Xu CT Proofread by Xu FM