Published online May 15, 2004. doi: 10.3748/wjg.v10.i10.1462
Revised: April 2, 2004
Accepted: April 10, 2004
Published online: May 15, 2004
AIM: To establish a novel human hepatocellular carcinoma (HCC) cell line FHCC-98 from HCC tissue and to provide a suitable model for studying HCC occurrence, progress and metastasis.
METHODS: Serially passaged cells were cultured and their morphologies were observed under light and electron microscope. Cytogenetic study was conducted by using flow cytometry and chromosome analysis. Expressions of tumor markers such as α-fetoprotein (AFP), cytokeratin (CK) and hepatoma metastasis-associated factor HAb18G/CD147 on the FHCC-98 cells were detected by immunocytochemistry or Western blotting. Lactic dehydrogenase (LDH) isoenzymes were detected by polyacrylamide gel electrophoresis (PAGE). Xenograft was performed by inoculating FHCC-98 cells into the flanks of nude mice.
RESULTS: Morphology of FHCC-98 cells was the same as that of other malignant cells. The expressions of the cells were positive for HAb18G/CD147 and CK, and negative for AFP. Its population doubling time was 21.4 h. The cell DNA was tetraploid and the major chromosomes were triploid by cytogenetics analysis. The tumorigenicity in nude mice was 100%. PAGE showed four bands representing LDH2, LDH3, LDH4 and LDH5.
CONCLUSION: FHCC-98 is a novel HCC cell line and an ideal cell model for further exploring the mechanism of hepatocellular carcinoma invasion and metastasis.
- Citation: Lou CY, Feng YM, Qian AR, Li Y, Tang H, Shang P, Chen ZN. Establishment and characterization of human hepatocellular carcinoma cell line FHCC-98. World J Gastroenterol 2004; 10(10): 1462-1465
- URL: https://www.wjgnet.com/1007-9327/full/v10/i10/1462.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i10.1462
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors. It ranks fifth in frequency worldwide among all malignancies and causes 1 million deaths annually[1], yet its incidence is increasing steadily in various countries[2-4]. Epidemiology studies showed that primary liver cancer is the second mortality in China[5] and it accounts for 53% of all liver cancer death worldwide[6]. Though with great development in diagnosis and therapy, the prognosis of patients with HCC remains dismal for its high rate of metastasis and recurrence. For patients in advanced stages, the median survival is less than 6 mo, no matter what kinds of therapy were managed[7-11]. So it is urgent to further explore the mechanism of HCC occurrence, progress and metastasis. HCC cell lines are powerful tools. Until now, ten of human HCC cell lines[12-27] have been established, and every cell line has its own characteristics and offers convenience for various experiments. Stability, homogeneity and easy to culture are important parameters for good cell lines. Here we report a new HCC cell line, which has been maintained for five years through 500 passages.
Tumor tissue obtained from a 39-year-old male HCC patient who lived in northwest China was used to establish a cell line. His family history of oncology was unknown, but there was no history of hepatitis or blood transfusions. There was no operation history. Investigations showed AST of 86 U/L, α-fetoprotein 8 ng/mL, carcinoembryonic antigen (CEA) 2.8 ng/mL and HBsAg(-). In 1998, the patient complained of upper right abdominal tender pain. A computerized-tomography demonstrated a 10 cm × 11 cm space-occupying lesion in the right lobe of liver. A partial liver resection was performed. Pathologic diagnosis confirmed HCC with middle differentiation. The patient died of lung metastasis after 3 mo.
A slice portion of sample was obtained under sterile conditions and washed by RPMI 1640 (Life Technology) 3 times and minced with surgical blades into pieces smaller than 1 mm3, then these pieces were put into tissue-culture flasks and incubated at 37 °C and 50 mL/L CO2 (Heraeus, Germany) with RPMI 1640 medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin and 200 mL/L FBS. One week later, cells migration was observed and some colonies were formed. Those colonies were detached with 2.5 g/L trypsin and pulled into 25 cm2 flasks in RPMI 1640 medium with 200 mL/L FBS. The medium was renewed two or three times a week. After stable growth, the same medium with 100 mL/L FBS was used as maintenance medium. When cells were confluent, they were detached with 2.5 g/L trypsin and passaged at the ratio of 1:2 or 1:3. At the 80th passage (about 30 mo after the sample was got), a stable cell line was considered to be established.
Cells were observed daily under an invert microscope (Olympus). Cells coated on tissue-culture slides were washed with PBS (pH7.4) 3 times, then fixed with cool pure acetone for 10 min and stained with hematoxylin and eosin (HE staining). For electron microscope observation, 4 × 106 cells were centrifuged at 1000 r/min for 10 min, then fixed in 30 g/L glutaraldehyde for 30 min and embedded in paraffin and ultra-thin sectioned. The section was observed with a JEM 2000 transmission electron microscope (JEOL)[22].
Growth curve was analyzed by modified MTT assay[24]. Cells in exponential growth phase at 16th passage were collected. Single-cell suspension (5 × 104/mL) was added to (200 μL/well) a 24-well plate, incubated at 37 °C with 50 mL/L CO2, then MTT (5 mg/mL) was added to the wells (20 μL/well) every 24 h for 7 consecutive days. The plate was incubated in cell incubator for 4 h, and then the supernatants were removed and 150 μL DMSO was added into each well. After incubation for 30 min, the dye-stained liquid was removed to a 96-well plate.
Absorbency at 490 nm (A490nm) was determined by M450 (Bio-Rad) enzyme-linked reader. The growth curve was plotted by half-height method.
FHCC-98 cells were incubated in another 24-well plate (l.2 × 104/mL). Attachment efficiency was measured by counting the cell number every 2 h.
Attachment efficiency = (number of cells/120000) × 100%.
To determine the cloning rate, 1.5 × 102 cells in suspension in a culture medium containing 8 g/L agar were applied to a base layer of a culture medium containing 1.7% agar. The Petri dish was incubated for 12 d at 37 °C in a humidified atmosphere containing 50 mL/L CO2, and colonies containing more than 5 cells were counted. Cloningrate = (Number of clones/150) × 100%
Single-cell suspensions containing 2 × 106 cells were treated following the standardized protocol and cell cycle analysis were performed by flow-cytometry (ELITE ESP Coulter)[22].
Cells were cultured for 2 h in the culture medium containing 0.05 μg/mL of colcemid and trypsinized, swollen with 0.075 mol/L KCl solution for 15 min and fixed with methanol: acetic acid (3:1) 3 times. Following air-drying, metaphase smears were stained with Giemsa. Samples were observed with oil immersion objective and photographed[18].
Cells cultured on slides for 24 h were washed 3 times with PBS and then fixed in acetone for 10 min at room temperature. AFP and cytokeratin were detected by immunocytochemistry using SP method. The slides were viewed under light microscope and the degree of staining was assessed.
Tumor metastasis-associated factor HAb18G/CD147[28,29] was detected by western blotting. Single-cell suspension (1.0 × 105/mL) was collected, and centrifuged at 800 r/min. The cell pellets were collected and resuspended in lysis buffer of 50 mmol/L Tris-HCl (pH8.0), 150 mmol/L NaCl, 0.2 g/L azido sodium, 1 g/L SDS, 100 μg/mL PMSF, 10 g/L Triton X-100, 5 g/L deoxycholic acid and protease inhibitor for 10 min on the ice. The lysis solution was centrifuged at 16000 g for 10 min at 4 °C and the supernatants were collected, and the protein concentration was detected with Lowery’s method. SDS-PAGE was carried out. Western blotting was performed with secondary antibodies coupled to horseradish peroxides and detected using ECL reagents (Amersham, Freiburg, German).
Single-cell suspension (5.0 × 105/mL) was mixed with the same volume of ConA and the mixture were diluted and incubated at 37 °C in 50 mL/L CO2 for 20 min. Human embryonic lung cell was used as contrast. Assembling reaction was observed.
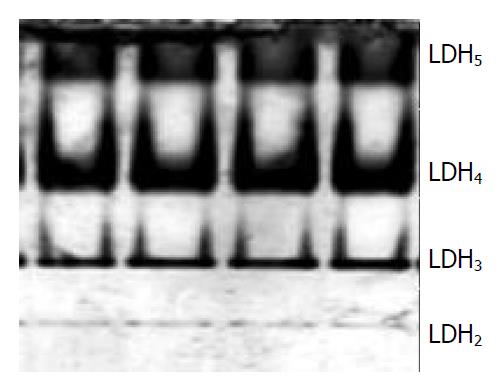
LDH isoenzymes were detected by PAGE. FHCC-98 cells were trypsinized and 8.0 × 106 cells were re-suspended in sterile saline. The cell suspension was centrifuged at 1000 r/min for 10 min twice and then re-suspended in 0.5 mL Tris-HCl (150 mmol/L, pH7.6). The sample was repeatedly frozen and thawed at -40 °C and 37 °C for 6 times, then centrifuged at 3000 r/min 4 °C for 20 min. The supernatant was stored under -20 °C for using. The SDS-PAGE sample buffer was 100 mmol/L Tris-HCl (pH6.8) supplemented with 200 mL/L glycerite and 2 g/L bromphenol blue. The sample was mixed with an equal volume of buffer and used for PAGE. The stacking gel was 50 g/L while separation gel 60 g/L. The staining solution was composed of oxidized NAD+ (5 mg/mL), NBT (2 mg/mL), PMS (1 mg/mL) and sodium lactate (60 g/L). The minigels were run at a constant voltage (100 V) for 2 h and then stained for 1 h. The gel was fixed in 70 mL/L acetic acid over 16 h and photographed.
Ten 4-6-week-old nude mice (supplied by the animal center of Fourth Military Medical University) of both sexes bred under specific pathogen-free (SPF) conditions were used in the heterotransplantation experiments. Cells (2.0 × 106) suspended in medium (0.2 mL) were injected subcutaneously into flanks of each mouse.
Mycoplasma was detected by transmission electron microscopy.
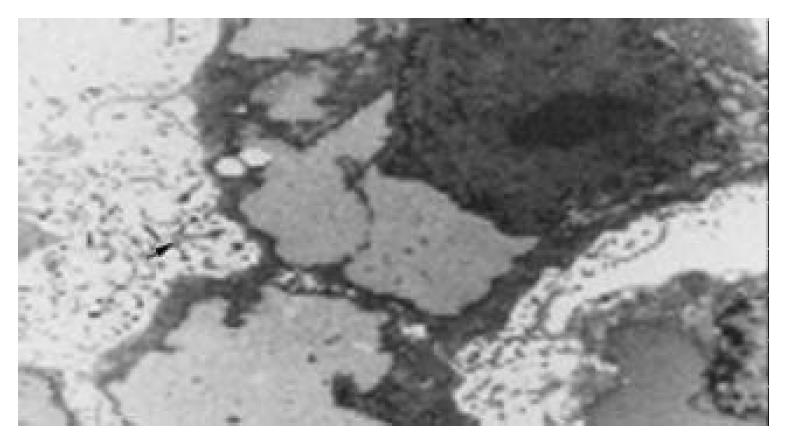
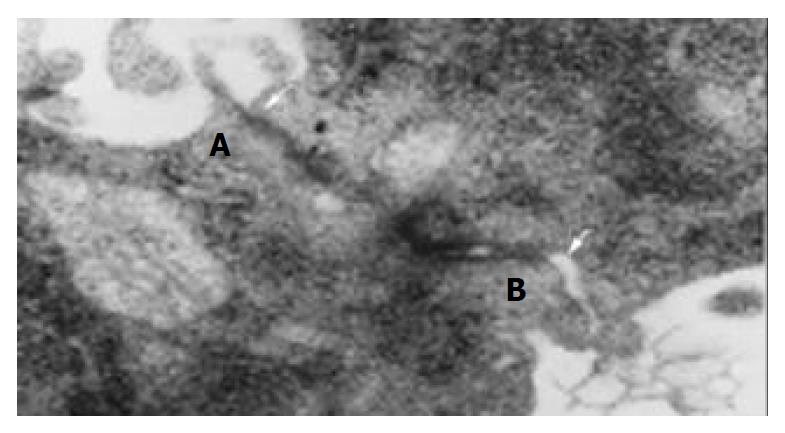
Polygonal epithelial-like cells in culture were identified to have large nuclei and the ratio of nuclear/cytoplasmic increased. Cells grew well at a high density (> 1 × 106/mL). Electron microscopy revealed that abundant microvilli distributed on the cell surface (Figure 1). Desmosomes and gap junction could be seen between cells (Figure 2). Glycogen granules were rich in cytoplasm and mitochondria were in a round shape. Ribosome and rough endoplasmic reticulum were moderate. Atypical nuclei were conspicuous and euchromatin was rich. Karyokinesis and pathologic mitosis were frequently uncoupled.
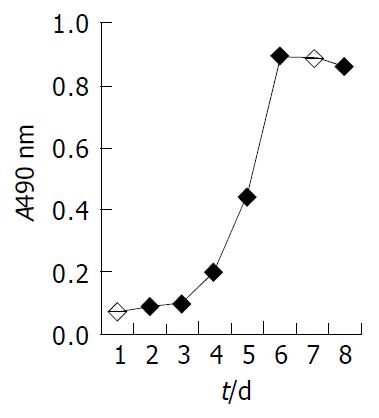
The growth curve at the sixteenth passage (Figure 3) showed a stationary-phase at the beginning and at the end of the experiment, the exponential growth lasted about 3 d. The population doubling time of FHCC-98 was 21.4 h. At the time points of 2, 4, 6 and 8 h, the attachment efficiencies were 54.2%, 83.3%, 89.2% and 93.7%, respectively. The percentage of colony-forming cells in soft agar was an average of 32.6%.
Chromosome analysis revealed the number of chromosomes per cell varied from 58 to 80 with a number in the triploid of 91%. FHCC-98 cell DNA became tetraploid. 75.0% cells were in G1 phase, while 7.0% cells were in G2 phase and 18.0% in S phase. The DNA index was 1.857.
The FHCC-98 cell line was positive for CK and negative for AFP.
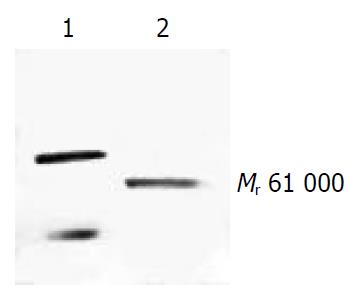
Western blotting showed that FHCC-98 was positive for HAb18G/CD147 and the band showed a single one with Mr 61000 (Figure 4).
The FHCC-98 cells were coagulated in 3.91 μg/mL of ConA while human embryonic lung cells had no response to 250 μg/mL of ConA.
PAGE analysis revealed that FHCC-98 had tumor typical isoenzymes of LDH without LDH1 (Figure 5). LDH2, LDH3, LDH4 and LDH5 isoenzymes were separated to relative activities (percentage of total LDH) of 3%, 19%, 49% and 29%, respectively.
Ten days after heterotransplantation of FHCC-98 cells into nude mice, subcutaneous tumors appeared at the sites of inoculation and grew rapidly in size during the next 20 d. Average tumor volumes on d 10, 14 and 30 were 42.4 ± 17.8, 76.6 ± 43.4, and 471.4 ± 187.5 mm3, respectively.
No mycoplasma contamination was detected in FHCC-98 cells from passages 16 and 28.
It is well known that heterogeneous tumors have diverse biologic behaviors, outcomes, and responses to therapy whether in vivo or in vitro. Malignant tumors show their genetic heterogeneities, differentiation and metastasis, which have been demonstrated in various kinds of tumors[30-34], and even in permanent cell lines with different biological characteristics[22,23,34]. Since the establishment of the first human HCC cell line in 1963 (Chen et al Chin Med J, 1963; 82: 228), more than ten human HCC cell lines have been established, most of which are positive for either HBV, HCV or AFP, yet they differed in biological properties. HBV is the main viral cause of HCC in China. Unfortunately, there are about 30% HCC patients are AFP or HBV negative, partially due to the extensive necrosis of tumor mass. As a tumor marker, AFP’s sensitivity and specificity for HCC detection depend on the diagnostic level and the cutoff level[35]. Different detection approaches may have different results. In this study, immunocytochemical method was applied to detect AFP and the result was consistent with that by RIA. Up to now, human HCC cell lines with both AFP negative and HBV negative have been rarely reported. Fortunately, we established a HCC cell line, which was negative for both AFP and HBV.
FHCC-98 demonstrated the same epithelial-type morphology as other HCC cell lines. FHCC-98 showed no contact inhibition when the cell density was high. As important metabolic parameters of HCC, LDH4 and LDH5 had high activities, and LDH1 had no, suggesting the existence of anaerobic respiration in HCC. Hypoxia and overgrowth of cancerous tissue specifically repressed the activity of AFP, and the target was c-Myc, suggesting that c-Myc regulated AFP gene expression[36]. Such changes in LDH5 might be a factor hindering the synthesis of AFP and resulting in AFP-negative patients. FHCC-98 may be used as a model for anti-oxidant therapy of tumors. FHCC-98 has its own characteristics. The first prominent characteristic of the cell line is its biological properties of high concordance and little variation among different passages including its morphology, growth kinetics, chromosome, AFP and LDH isoenzyme; The second one is that its population doubling time was relatively shorter and it grew more rapidly compared with other human HCC cell lines[12,14,18,20,23,25]. High coagulation of the cells showed that it could proliferate rapidly without contact inhibition. The third one is its high tumorigenicity in nude mice. And the last one of the cell line is that it can grow in various medium cultures, such as RPMI 1640 and DMEM with different concentrations of FBS.
In conclusion, we believe that FHCC-98 is a novel HCC cell line and a valuable cell model for studying HCC in vitro and in vivo, especially for AFP- and HBV-negative HCC.
| 1. | Yu AS, Keeffe EB. Management of hepatocellular carcinoma. Rev Gastroenterol Disord. 2003;3:8-24. [PubMed] |
| 2. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2135] [Article Influence: 79.1] [Reference Citation Analysis (1)] |
| 3. | Kiyosawa K, Tanaka E. Characteristics of hepatocellular carcinoma in Japan. Oncology. 2002;62 Suppl 1:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. [PubMed] |
| 5. | Zhang S, Li L, Lu F. [Mortality of primary liver cancer in China from 1990 through 1992]. Zhonghua Zhongliu Zazhi. 1999;21:245-249. [PubMed] |
| 6. | Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the world-wide mortality from 25 cancers in 1990. Int J Cancer. 1999;83:18-29. [RCA] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 7. | Johnson PJ. Hepatocellular carcinoma: is current therapy really altering outcome? Gut. 2002;51:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Watanabe T, Omori M, Fukuda H, Takada H, Miyao M, Mizuno Y, Ohsawa I, Sato Y, Hasegawa T. Analysis of sex, age and disease factors contributing to prolonged life expectancy at birth, in cases of malignant neoplasms in Japan. J Epidemiol. 2003;13:169-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Ziparo V, Balducci G, Lucandri G, Mercantini P, Di Giacomo G, Fernandes E. Indications and results of resection for hepatocellular carcinoma. Eur J Surg Oncol. 2002;28:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Kanematsu T, Furui J, Yanaga K, Okudaira S, Shimada M, Shirabe K. A 16-year experience in performing hepatic resection in 303 patients with hepatocellular carcinoma: 1985-2000. Surgery. 2002;131:S153-S158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Aguayo A, Patt YZ. Liver cancer. Clin Liver Dis. 2001;5:479-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Yano H, Maruiwa M, Murakami T, Fukuda K, Ito Y, Sugihara S, Kojiro M. A new human pleomorphic hepatocellular carcinoma cell line, KYN-2. Acta Pathol Jpn. 1988;38:953-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Dor I, Namba M, Sato J. Establishment and some biological characteristics of human hepatoma cell lines. Gan. 1975;66:385-392. [PubMed] |
| 14. | Alexander JJ, Bey EM, Geddes EW, Lecatsas G. Establishment of a continuously growing cell line from primary carcinoma of the liver. S Afr Med J. 1976;50:2124-2128. [PubMed] |
| 15. | Tang ZY, Sun FX, Tian J, Ye SL, Liu YK, Liu KD, Xue Q, Chen J, Xia JL, Qin LX. Metastatic human hepatocellular carcinoma models in nude mice and cell line with metastatic potential. World J Gastroenterol. 2001;7:597-601. [PubMed] |
| 16. | Murakami T, Yano H, Maruiwa M, Sugihara S, Kojiro M. Establishment and characterization of a human combined hepatocholangiocarcinoma cell line and its heterologous transplantation in nude mice. Hepatology. 1987;7:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Saito H, Morizane T, Watanabe T, Kagawa T, Iwabuchi MN, Kumagai N, Inagaki Y, Tsuchimoto K, Tsuchiya M. Establishment of a human cell line (HCC-T) from a patient with hepatoma bearing no evidence of hepatitis B or A virus infection. Cancer. 1989;64:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Sing GK, Pace R, Prior S, Scott JS, Shield P, Martin N, Searle J, Battersby C, Powell LW, Cooksley WG. Establishment of a cell line from a hepatocellular carcinoma from a patient with hemochromatosis. Hepatology. 1994;20:74-81. [PubMed] [DOI] [Full Text] |
| 19. | Le Jossic C, Glaise D, Corcos L, Diot C, Dezier JF, Fautrel A, Guguen-Guillouzo C. trans-Acting factors, detoxication enzymes and hepatitis B virus replication in a novel set of human hepatoma cell lines. Eur J Biochem. 1996;238:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Lee JH, Ku JL, Park YJ, Lee KU, Kim WH, Park JG. Establishment and characterization of four human hepatocellular carcinoma cell lines containing hepatitis B virus DNA. World J Gastroenterol. 1999;5:289-295. [PubMed] |
| 21. | Seki S, Kitada T, Kawada N, Sakaguchi H, Kadoya H, Nakatani K, Satake K, Kuroki T. Establishment and characteristics of human hepatocellular carcinoma cells with metastasis to lymph nodes. Hepatogastroenterology. 1999;46:2812-2817. [PubMed] |
| 22. | Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY, Chen J, Xue Q. New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. Br J Cancer. 1999;81:814-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 225] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 23. | Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, Chen J, Gao DM, Bao WH. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7:630-636. [PubMed] |
| 24. | Yang JX, Tang WX. [Establishment of a cisplatin-induced human hepatocellular carcinoma drug-resistant cell line and its biological characteristics]. Aizheng. 2002;21:872-876. [PubMed] |
| 25. | Wu X, Wang Z, Liu B, Liu J, Gao Y, Li Z, Liu C. [Establishment and characterization of human extrahepatic growing hepatocellular carcinoma cell line EGHC-9901]. Zhonghua Waike Zazhi. 2002;40:616-617. [PubMed] |
| 26. | Li Y, Tang Z, Ye S, Liu Y, Chen J, Xue Q, Huang X, Chen J, Bao W, Yang J. [Establishment of human hepatocellular carcinoma cell line with spontaneous pulmonary metastasis through in vivo selection]. Zhonghua Yixue Zazhi. 2002;82:601-605. [PubMed] |
| 27. | Wen JM, Huang JF, Hu L, Wang WS, Zhang M, Sham JS, Xu JM, Zeng WF, Xie D, Liang LJ. Establishment and characterization of human metastatic hepatocellular carcinoma cell line. Cancer Genet Cytogenet. 2002;135:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Jiang JL, Zhou Q, Yu MK, Ho LS, Chen ZN, Chan HC. The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J Biol Chem. 2001;276:46870-46877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Li Y, Shang P, Qian AR, Wang L, Yang Y, Chen ZN. Inhibitory effects of antisense RNA of HAb18G/CD147 on invasion of hepatocellular carcinoma cells in vitro. World J Gastroenterol. 2003;9:2174-2177. [PubMed] |
| 30. | Kim GJ, Cho SJ, Won NH, Sung JM, Kim H, Chun YH, Park SH. Genomic imbalances in Korean hepatocellular carcinoma. Cancer Genet Cytogenet. 2003;142:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Shindo-Okada N, Takeuchi K, Nagamachi Y. Establishment of cell lines with high- and low-metastatic potential from PC-14 human lung adenocarcinoma. Jpn J Cancer Res. 2001;92:174-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Tammen H, Kreipe H, Hess R, Kellmann M, Lehmann U, Pich A, Lamping N, Schulz-Knappe P, Zucht HD, Lilischkis R. Expression profiling of breast cancer cells by differential peptide display. Breast Cancer Res Treat. 2003;79:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Nelson SJ, Cha S. Imaging glioblastoma multiforme. Cancer J. 2003;9:134-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Yang J, Qin LX, Ye SL, Liu YK, Li Y, Gao DM, Chen J, Tang ZY. The abnormalities of chromosome 8 in two hepatocellular carcinoma cell clones with the same genetic background and different metastatic potential. J Cancer Res Clin Oncol. 2003;129:303-308. [PubMed] |
| 35. | Mazure NM, Chauvet C, Bois-Joyeux B, Bernard MA, Nacer-Chérif H, Danan JL. Repression of alpha-fetoprotein gene expression under hypoxic conditions in human hepatoma cells: characterization of a negative hypoxia response element that mediates opposite effects of hypoxia inducible factor-1 and c-Myc. Cancer Res. 2002;62:1158-1165. [PubMed] |
| 36. | Taketa K, Okada S, Win N, Hlaing NK, Wind KM. Evaluation of tumor markers for the detection of hepatocellular carcinoma in Yangon General Hospital, Myanmar. Acta Med Okayama. 2002;56:317-320. [PubMed] |
Co-correspondents: Zhi-Nan Chen
Edited by Chen WW and Xu FM