Published online May 15, 2004. doi: 10.3748/wjg.v10.i10.1409
Revised: November 29, 2003
Accepted: December 6, 2003
Published online: May 15, 2004
AIM: To investigate the inhibitory effects of endostatin-vascular endothelial growth inhibitor (VEGI151) recombinant adenoviruses on neovascularization.
METHODS: We used recombinant adenoviruses to treat human vascular endothelial cell line ECV304, human hepatocellular carcinoma cell line HepG2, and murine fibroblast cell line L929, in order to study the chimeric gene expression in these cell lines. Chick choriallantic membrane (CAM) model, rabbit inflammatory corneal neovascularization (CNV) model, and liver cancer-bearing nude mice model were employed to investigate the negative biological effect of fusion molecules on neovascularization in vivo.
RESULTS: Western blot showed that the molecular weight of fusion protein was about 41kD after infection of ECV304, HepG2 and L929 cells with supernatant of AdhENDO-VEGI151. The fusion protein showed a specific inhibitory effect on the proliferation of ECV304 cells, but no inhibitory effect on the growth of HepG2 and L929 cells (F = 13112.13, P = 0.0001). In the chick choriallantic membrane (CAM) assay, the expressed fusion protein significantly inhibited neovascularization. Rabbit inflammatory corneal neovasculari-zation (CNV) induced by intrastromal sutures resulted in a uniform neovascular response. In this model, direct subconjunctival injection of AdhENDO-VEGI151 expressed the fusion protein in vivo and suppressed the development of CNV. Topical application of AdhENDO-VEGI151 led to a significant suppression of CNV (F = 1413.11, P = 0.0001), as compared with the control group of AdLacZ. Immunohist-ochemical staining showed the fusion protein dominantly expressed in corneal epithelium. Compared with the control group of AdLacZ (4075.9 ± 1849.9 mm3), the average tumor size of group AdhENDO-VEGI151 reduced in size (487.7 ± 241.2 mm3) (F = 14.80, P = 0.0085), with an inhibition rate of 88.03%. Immunohistochemical staining showed the adenoviruses carried the fusion gene expressed on liver cancer cell membrane. MVD decreased more significantly in treated mice (30.75% ± 3.31%) than in AdLacZ control (50.25% ± 8.65%) (F = 17.72, P = 0.0056) with an inhibition rate of 39%.
CONCLUSION: Fusion protein expressed by recombinant adenoviruses has a significant inhibitory effect on neovasculari-zation.
- Citation: Pan X, Wang Y, Zhang M, Pan W, Qi ZT, Cao GW. Effects of endostatin-vascular endothelial growth inhibitor chimeric recombinant adenoviruses on antiangiogenesis. World J Gastroenterol 2004; 10(10): 1409-1414
- URL: https://www.wjgnet.com/1007-9327/full/v10/i10/1409.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i10.1409
Neovascularization plays a critical role in solid tumor growth and corneal opacification. Researches indicated that angiogenesis inhibitors were highly potent in suppressing angiogenesis which could enable the tumor mass to remain in a dormant state[1-4] and inhibit the development of corneal neovascularization[5-7]. Endostatin could cause G (1) cell cycle arrest in endothelial cells, inhibit endothelial cell proliferation and migration, and promote apoptosis[8]. Vascular endothelial cell growth inhibitor (VEGI) belongs to the tumor necrosis factor superfamily. VEGI is another endothelial cell-specific gene and a potent inhibitor of endothelial cell proliferation, angiogenesis[9]. VEGI could mediate early G (1) arrest in G (0)/G (1) endothelial cells responding to growth stimuli, and programmed death in proliferating endothelial cells[10]. In our laboratory two recombinant proteins have been used to treat tumor-bearing nude mice, but the inhibitory effect was not satisfactory. A few groups have demonstrated that antiangiogenic gene therapy with viral vectors is a potentially useful approach for inhibiting tumor growth in mouse models[11-15]. In the current study, we acquired recombinant adenoviruses carrying endostatin-vascular endothelial growth inhibitor fusion gene to investigate its inhibitory effect in vitro on endothelial cells and anti-angiogenic activity in vivo on chicken chorioallantoic membrane, inflammatory corneal neovascularization (CNV) in rabbit and liver cancer in nude mice.
AdhENDO-VEGI151 and AdLacZ were prepared in our laboratory. Endostatin polyclonal antibody was a gift from Dr. Stanker (Hanover, Germany). VEGI polyclonal antibody was prepared in our laboratory. Ad5-transformed human embryo renal cell line 293, human vascular endothelial cell line ECV304, human hepatocellular carcinoma cell line HepG2, and murine fibroblast cell line L929 were purchased from the Institute of Cell Biology, Chinese Academy of Sciences. Cell culture media were obtained from GIBCO. Nine-day-old fertilized white Leghorn eggs, New Zealand white rabbits, liver cancer-bearing nude mice SMMC-LTNM, and BALB/c nude mice were purchased from the Center of Experiment Animals, Second Military Medical University (Shanghai, China). All cell lines and animals were maintained under standard conditions.
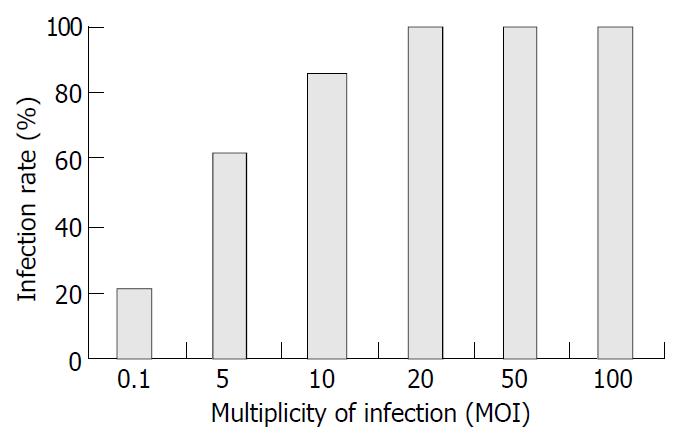
Recombinant adenovirus infectivity examination AdhENDO-VEGI151 and AdLacZ were acquired as described previously[16,17]. In a 24-well plate, 6 × 105 293 cells were infected with 50 μL of identified recombinant adenoviruses AdhENDO-VEGI151 or AdLacZ in 150 μL of Dulbecco’s modified Eagle’s medium (DMEM) containing 100 mL/L fetal bovine serum (200 μL in total) for 72 h. The cells showed evidence of cytopathic effect (CPE) and its supernatant was harvested. After three freeze/thaw cycles, the mixtures were passed through a 0.45 μm millipore filter. The filtrate was frozen at -70 °C and stored. TCID50 was used to determine the titer of recombinant adenoviruses[18]. Ten thousand ECV304 cells were incubated in a 96-well plate for 5 h. Cells were infected with AdLacZ, covering a proper multiplicity of infection (MOI) range (0.1, 5, 10, 20, 50 and 100 MOI), and incubated for 48 h. X-gal staining was performed and blue stained cells were counted under a microscope.
Western blotting analysis Ad5-transformed human embryo renal cell line 293, human vascular endothelial cell line ECV304, human hepatocellular carcinoma cell line HepG2, and murine fibroblast cell line L929 were used in this assay. In a 6-well plate, 1 × 106 cells were infected with recombinant adenoviruses at an MOI of 20 for 4 h, then the medium containing recombinant adenoviruses was replaced with 1 mL of normal medium and cells were incubated for 24 h. Cell extracts were separated by 100 g/L sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions and electroblotted onto nitrocellulose membrane followed by treatment with blocking buffer containing 50 g/L nonfat dry milk in TBST (20 mmol/L Tris [pH8.0], 150 mmol/L NaCl, 1 g/L Tween-20) for 1 h. The electroblotted membranes were incubated with rabbit polyclonal antibodies against VEGI proteins, then subsequently incubated with peroxidase horseradish-conjugated anti-rabbit immunoglobulin for 1 h at 37 °C. The immune complexes were visualized with diaminobenzidine (DAB) staining.
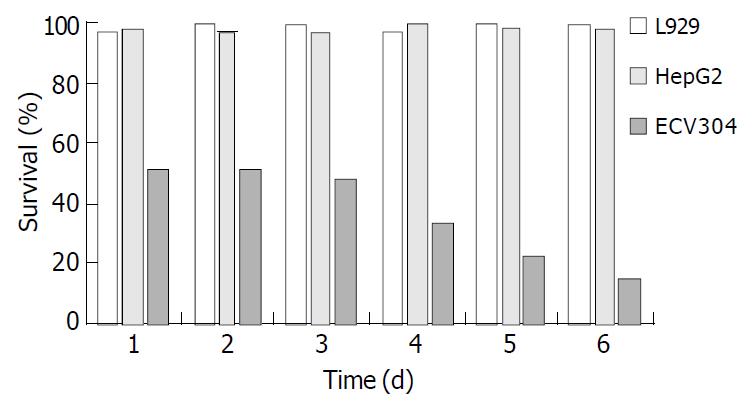
In vitro bioactivity of fusion gene products 1 × 103 cells were incubated in the 96-well plates, cells were infected with recombinant adenoviruses at an MOI of 20 for 4 h. The cell lines were ECV304, HepG2, and L929, respectively. The recombinant adenoviruses were AdhENDO-VEGI151 and AdLacZ, respectively. The medium containing recombinant adenoviruses was replaced with 100 μL of normal medium and cells were incubated at 37 °C for an appropriate period of time. The medium containing recombinant adenoviruses was removed, murine fibroblast cell line L929 was incubated with a normal medium containing 0.5 μg/mL actinomycin D. Cell viability was determined by crystal violet vital staining[19]. Cell viability (%) = (AdhENDO-VEGI151 infected group A570/630/AdLacZ control group A570/630) × 100.
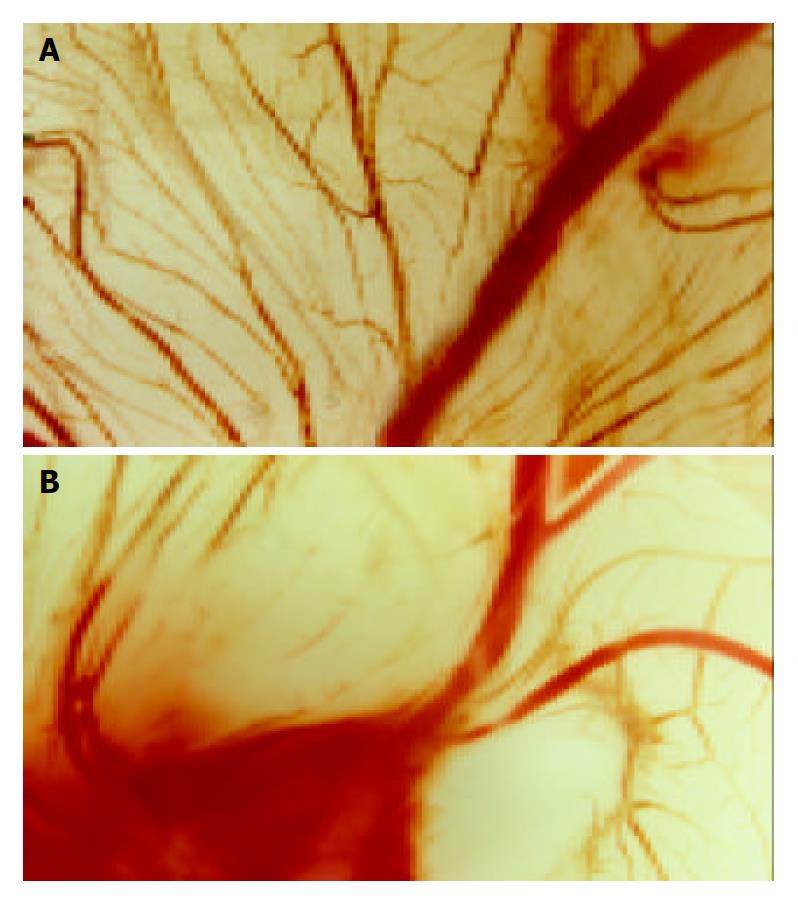
Inhibition of chick embryonic chorioallantoic membrane angiogenesis by recombinant adenoviruses A 1 cm × 1 cm window in the shell was drilled over the air sac at the end of 9-day-old eggs. Chick embryonic chorioallantoic membrane was exposed by tearing up the egg membrane with a tip. A disk of methylcellulose was put on CAM, and 10 μL recombinant adenoviruses was added at a titer of 1 × 1012 TCID50/L. The windows were sealed with aseptic tapes and the eggs were incubated for 3 d. The membrane was cut around the air sac, which was turned upside down and observed by a stereomicroscope.
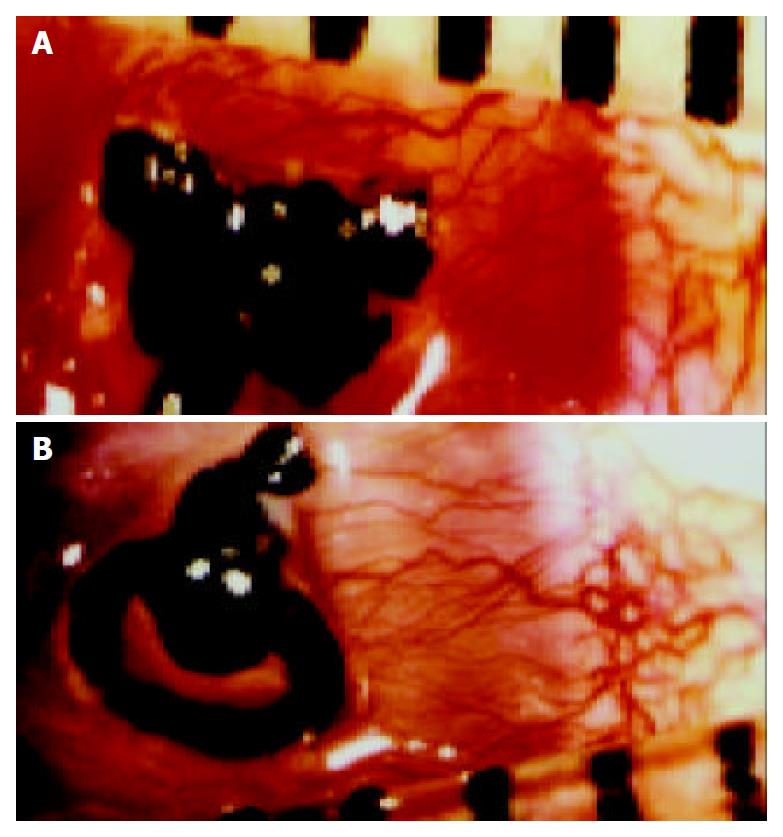
Suppression of rabbit inflammatory corneal neovascularization In this study, 4 female New Zealand white rabbits, weighing 4-5 kg, were used. Inflammatory CNV in rabbits induced by placement of intrastromal sutures resulted in a uniform neovascular response as described[20]. On the first day after suturation, four rabbits were divided into AdlacZ control group and AdhENDO-VEGI151 group. One hundred microliters of each recombinant adenovirus at a titer of 1 × 1012 TCID50/L was injected into subconjunctiva. Then, gentamicin ointment was applied to the eyes once per day. Eyes were examined daily by a slit-lamp biomicroscope and a surgical microscope was used to monitor angiogenic responses to recombinant adenoviruses. The area of corneal neovascularization was determined by measuring the vessel length (L) from the limbus and the number of clock hours (C) of limbus involved[21-24]. Only the uniform contiguous band of neovascularization adjacent to the suture was measured. A formula was used to determine the area[25]: C/12 × 3.1416 × [r2 - (r-L)2], where r = 6 mm (the measured radius of rabbit cornea). Rabbits were sacrificed on d 21. Rabbits’ eyes were enucleated and inflammatory cornea was fixed in 40 g/L paraformaldehyde for 24 h.
Immunohistochemical staining of fusion protein in inflammatory cornea of rabbits Buffered formaldehyde fixed tissues were embedded in paraffin, 4-μm thick sections were treated with 3 mL/L H2O2 in methanol for 20 min, and blocked with normal rabbit serum (1:20 dilution) for 20 min at room temperature. Then, the sections were incubated with rabbit polyclonal anti-endostatin (1:50 dilution) for 1 h at room temperature, followed by incubation with biotinylated goat anti-rabbit antibody IgG for 30 min and horseradish peroxidase-conjugated streptavidin at room temperature for 30 min. After completion of conjugation reaction, the slides were stained using 3,3’-diaminobenzidine (DAB)-H2O2. The sections were counterstained with hematoxylin, and viewed using a light microscope.
Treatment of liver cancer-bearing nude mice with recombinant adenoviruses Three SMMC-LTNM nude mice with liver cancer were sacrificed, and skin overlying the tumor was cleaned with ethanol. The tumors were resected under aseptic conditions. A suspension of tumor cells in Hank’s was made by passage of viable tumor tissues small hypodermic needles. The final concentration was adjusted to 2 × 107 cells/mL. Female Balb/c nude mice, 3 wk of age, received injections sc into the dorsal midline in a 200 μL volume. Tumors appeared approximately 10 d after implantation. The animals were randomized into 2 groups, and each group had four mice with comparable tumor size within and among the groups. Five hundred microliters of recombinant adenoviruses AdhENDO-VEGI151 or AdLacZ at a titer of 1 × 1012 TCID50/L was administered 10 times by sc every other day. The mice were sacrificed 24 h after the last injection, tumors from each mouse were removed and fixed in 40 g/L neutral buffered formaldehyde. Tumor size in all groups was measured, tumor volume was determined using the formula π× width2× length/6. Tumor weight, inhibition rate and expression of target gene were evaluated, respectively.
Immunohistochemical staining of fusion protein in liver cancer of nude mice Tissues from liver cancer of nude mice were fixed in 40 g/L neutral buffered formaldehyde, embedded in paraffin, and sectioned into 4 μm. The sections were treated with PBS, blocked with normal rabbit serum, incubated with rabbit polyclonal anti-endostatin (1:100 dilution) overnight at 4 °C, washed with PBS, incubated with HRP-conjugated goat anti-rabbit antibody (1:500 dilution) for 1 h at 37 °C, and stained using 3,3’-diaminobenzidine (DAB)-H2O2, counterstained with hematoxylin, and examined by a light microscope.
Determination of intratumor microvessel density Determination of microvessel density was carried out as described[26-28]. Briefly, intratumor microvessels were immunostained with rat anti-human CD34 monoclonal antibody, and visualized with a biotinylated anti-rat IgG antibody by the Strept Avidin-Biotin Complex method. The sections were examined under low power to identify most vascular areas of the tumor. Within these areas, a maximum of 3 fields at × 200 magnification was examined, and the mean values were calculated.
Statistical analysis Data were expressed as mean ± SD. An analysis of variance was performed with Statview 4.0 statistical analysis software, and P < 0.05 was considered statistically significant.
The titer of amplification of these two kinds of recombinant adenoviruses was 1 × 1012 TCID50/L. When ECV304 cells were infected with AdLacZ at an MOI of 0.1, 20% cells showed LacZ-positive staining. If MOI was 20, 100% cells were stained blue (Figure 1). This result suggested recombinant adenoviruses had the highly efficient gene delivery
Four kinds of cell lines infected with AdhENDO-VEGI151 were immunoblotting stained with polyclonal antibodies against VEGI and visualized with DAB system. The results demonstrated that the fusion gene carried by AdhENDO-VEGI151 could be in vitro expressed in all the cell lines, and the specific bands were located at about 41 ku, which was the expected size of ENDO-VEGI151 fusion protein (Figure 2).
Human vascular endothelial cells ECV304 were sensitive to AdhENDO-VEGI151, and cell proliferation was significantly inhibited. In contrast, no inhibitory activities on HepG2 and L929 in vitro growth were observed following AdhENDO-VEGI151 infection (Figure 3). Further analysis of variance showed there was a significant difference in viability between ECV304 cells and non-endothelial cells (HepG2 cells and L929 cells) (F = 13112.13, P = 0.0001). At different time points, there was a significant difference between the two groups (F = 72.75, P = 0.0001). There was no significant difference in viability between HepG2 cells and L929 cells neither for the hypothesis of no time effect (F = 7.17, P = 0.0554) nor at different time point (F = 1.74, P = 0.2424).
Nine-day old chick embryos were incubated with filter disks infected with AdhENDO-VEGI151, or AdLacZ. The effect of AdhENDO-VEGI151 on CAM angiogenesis was analyzed 3 d after incubation, by excising the membrane around the air sac and microscopy (Figure 4). At doses of 10-20 μL/disc at a titer of 1 × 1012 TCID50/L, there was a potent inhibition of angiogenesis of AdhENDO-VEGI151 on the tested CAMs (n = 4/group). In contrast, AdLacZ failed to suppress angiogenesis in CAM. There was no evidence of toxicity in any chick embryos tested.
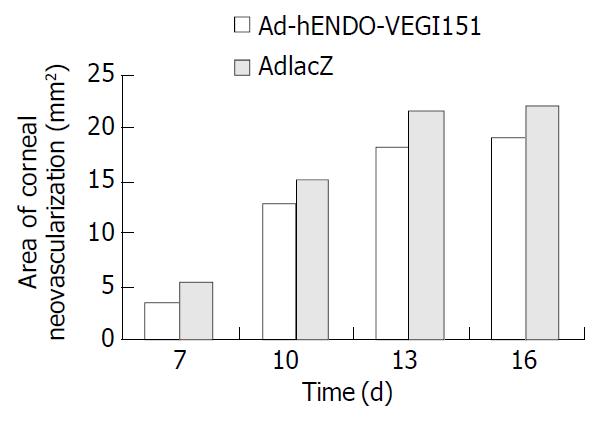
After direct subconjunctival injection of recombinant adenoviruses, rabbit eyes were examined by a surgical microscope daily. A few small capillary buds that arose from engorged limbal arcades were observed. CNV increased gradually, peaked on d 12-14, and degenerated on day 15 after suture induction. So we chose day 7, 10, 13, or 16 as time points to determine the number and length of vessels, and the area of neovascularization. Analysis of the CNV area found that local application of AdhENDO-VEGI151 resulted in a significant suppression of CNV (Figure 5, Figure 6). Repeated measurement of two factors and multilevel analysis of variance showed there were significant differences between AdhENDO-VEGI151 group and AdlacZ group (F = 1413.11, P = 0.0001) for the hypothesis of no time effect. At different time points, there was a significant difference between the two groups (F = 15517.87, P = 0.0001).
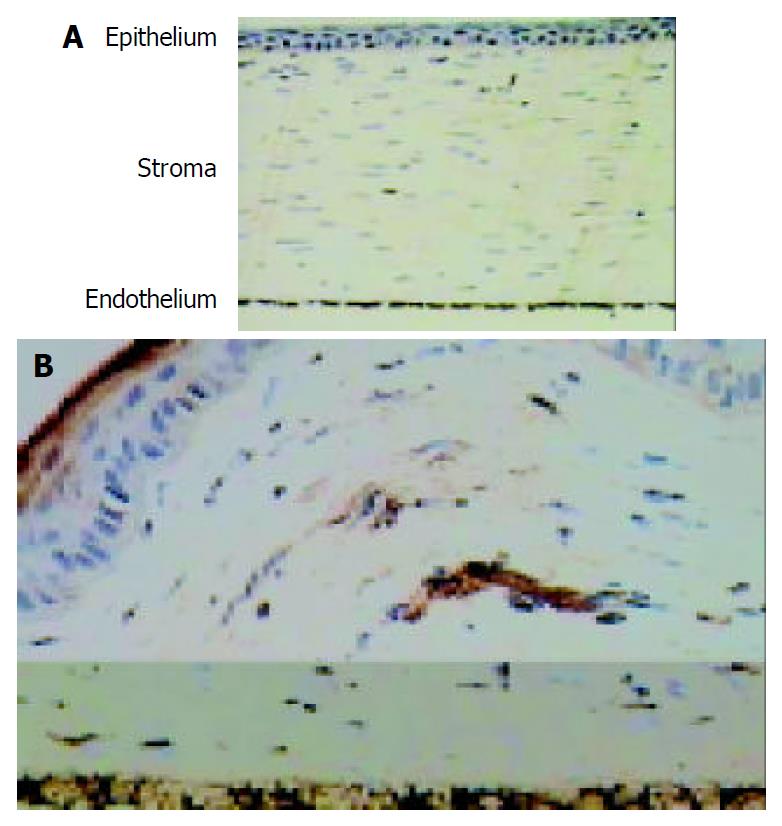
The fusion gene expression in inflammatory cornea of rabbits was detected by immunohistochemistry. Corneal endothelium was stained brown in AdLacZ group, others were stained negative. Corneal endothelium and epithelium were stained brown in AdhENDO-VEGI151 group (Figure 7), and lasted for 21 d.
Twenty-one days after therapy, the mice were sacrificed. Compared with AdLacZ control group (4075.9 ± 1849.9 mm3), the average tumor size of AdhENDO-VEGI151 group had confirmed regression (487.7 ± 241.2 mm3) (F = 14.80, P = 0.0085) with an inhibition rate of 88.03%.
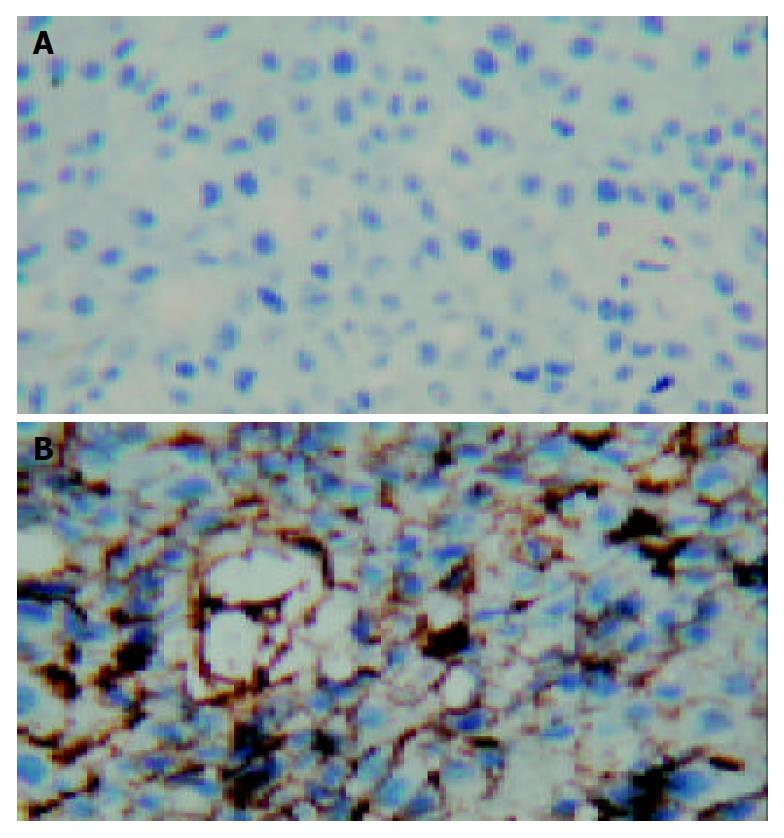
When we examined the sections stained by rabbit polyclonal anti-endostatin by a light microscope, strongly positive staining of fusion molecules presented in all cases of animals treated with AdhENDO-VEGI151. The recombinant adenoviruses carrying chimeric gene could be expressed in liver cancer. Brown staining was mainly located on the membrane of liver cancer cells (Figure 8). There was no positive staining in tumor tissues of the control animals.
The antiangiogenesis effect of fusion protein producing adenoviruses was evaluated in tumor model. Tumor microvessel density (MVD) was reduced by the production of hENDO-VEGI151 fusion gene. The MVD was decreased (30.75% ± 3.31%) more significantly in treated mice than in control (50.25% ± 8.65%) (F = 17.72, P = 0.0056).
Endostatin and vascular endothelial growth inhibitor are endogenous angiostatic molecules. Both of them are angiogenesis inhibitors that could suppress the growth of endothelial cells[29,30]. To achieve effectiveness, antiangiogenic therapy with endostatin or VEGI in tumor-bearing mice required prolonged administration and high doses of recombinant proteins[31,32]. Production of functional polypeptides has proven difficult, perhaps because of its physical properties or variations in the purification procedures in different laboratories[33,34]. In our laboratory (Department of Microbiology, Second Military Medical University, Shanghai, China) these two recombinant proteins were used respectively to treat tumor-bearing nude mice, but their bioactivities were not satisfactory. So we performed antiangiogenic gene therapy with adenoviral vectors. The results suggested recombinant adenoviruses AdhENDO-VEGI151 could express fusion protein about 41 ku in ECV304, HepG2, L929 and 293 cells, and showed a specific inhibition on the proliferation of ECV304 cells, the inhibition rate reached 49% in 24 h and 86% in 144 h, respectively. But the AdhENDO-VEGI151 showed no suppression on growth of HepG2 cells. VEGI belongs to the tumor necrosis factor (TNF) superfamily[35] and was constitutively expressed in endothelial cells. Since murine fibroblast cell line L929 was used to determine the bioactivity of TNF, we also used this cell line to test the bioactivity of AdhENDO-VEGI151. The results showed it had no suppression on growth of L929 cells, suggesting that fusion protein expressed by AdhENDO-VEGI151 infection had no cytotoxicity to non-endothelial cells. Chick choriallantic membrane assay showed that fusion protein expressed by AdhENDO-VEGI151 infection could significantly inhibit angiogenesis, and form no vessel regions. Because CAM was formed during embryogenic period, it was difficult to distinguish new vessels from previously established vascular networks. On the other hand, inflammatory CNV assay could avoid any confusion between new vessels and previously existed vessels, and any vessels penetrating into the corneal stroma could be identified as newly formed, as the cornea was avascular. For CNV was the main reason for corneal opacification, and also the main risk factor of immunologic rejection in allogenous corneal transplantation, we tried to use AdhENDO-VEGI151 as a safe and effective agent to inhibit CNV. We used intrastromal sutures to induce inflammatory CNV in rabbits. Neovascularization began sprouting into the corneal stroma from the limbus to the site of sutures within a few days after direct subconjunctival injection. Since small capillary vessels subsided gradually 16 d after suture induction, we selected day 7, 10, 13, or 16 as time points to measure vessel length and clock hours of circumferential neovascularization. Analysis of CNV area found there were significant differences between AdhENDO-VEGI151 group and AdlacZ group. Local application of AdhENDO-VEGI151 led to significant suppression of CNV, the inhibition rate was 36%, 16%, 17%, or 13% in turn. Our results suggested that in order to sustain the angiogenesis inhibition, AdhENDO-VEGI151 should be administrated every 7 d. Immunohistochemical staining showed that the positive reaction was predominant in corneal epithelium of AdhENDO-VEGI151 group. These results suggested that the expression of recombinant adenoviruses was related with the site of injection. Positive staining in corneal endothelium was observed in two groups. This phenomenon might be caused by the endogenous endostatin expression of corneal endothelial cells. Compared with AdLacZ control group, the average tumor size of AdhENDO-VEGI151 group was reduced with an inhibition rate of 88.03%. The gene of interest in adenoviruses could express in liver cancer. Since the fusion gene included IL-3 signal peptide coding sequence, the fusion protein might secrete out of the membrane of liver cancer cells. MVD was decreased more significantly in treated mice than that in control, the inhibition rate was 39%. These findings in combination with the results of cell assay suggested that the antitumor activity of AdhENDO-VEGI151 was not due to a direct effect on tumor cells, interference with the development of tumor microvessel density was responsible for tumor regression. However, up to now, we do not know whether the combination is more potent or not. Comparison of the effectiveness of fusion molecules with individual molecules has been under investigation.
| 1. | Kleinman HK, Liau G. Gene therapy for antiangiogenesis. J Natl Cancer Inst. 2001;93:965-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Tomanek RJ, Schatteman GC. Angiogenesis: new insights and therapeutic potential. Anat Rec. 2000;261:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Kim CW, Lee HM, Lee TH, Kang C, Kleinman HK, Gho YS. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002;62:6312-6317. [PubMed] |
| 4. | Oehler MK, Bicknell R. The promise of anti-angiogenic cancer therapy. Br J Cancer. 2000;82:749-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Lai CM, Brankov M, Zaknich T, Lai YK, Shen WY, Constable IJ, Kovesdi I, Rakoczy PE. Inhibition of angiogenesis by adenovirus-mediated sFlt-1 expression in a rat model of corneal neovascularization. Hum Gene Ther. 2001;12:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Lai CM, Spilsbury K, Brankov M, Zaknich T, Rakoczy PE. Inhibition of corneal neovascularization by recombinant adenovirus mediated antisense VEGF RNA. Exp Eye Res. 2002;75:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Ambati BK, Joussen AM, Ambati J, Moromizato Y, Guha C, Javaherian K, Gillies S, O'Reilly MS, Adamis AP. Angiostatin inhibits and regresses corneal neovascularization. Arch Ophthalmol. 2002;120:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Hanai J, Dhanabal M, Karumanchi SA, Albanese C, Waterman M, Chan B, Ramchandran R, Pestell R, Sukhatme VP. Endostatin causes G1 arrest of endothelial cells through inhibition of cyclin D1. J Biol Chem. 2002;277:16464-16469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Zhang M, Wang L, Wang HW, Pan X, Pan W, Qi ZT. [Effect of N-terminal deletion on biological activity of vascular endothelial cell growth inhibitor]. Shengwu Huaxue Yu Shengwu Wuli Xuebao (Shanghai). 2003;35:133-137. [PubMed] |
| 10. | Yu J, Tian S, Metheny-Barlow L, Chew LJ, Hayes AJ, Pan H, Yu GL, Li LY. Modulation of endothelial cell growth arrest and apoptosis by vascular endothelial growth inhibitor. Circ Res. 2001;89:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Tanaka T, Cao Y, Folkman J, Fine HA. Viral vector-targeted antiangiogenic gene therapy utilizing an angiostatin complementary DNA. Cancer Res. 1998;58:3362-3369. [PubMed] |
| 12. | Hampl M, Tanaka T, Albert PS, Lee J, Ferrari N, Fine HA. Therapeutic effects of viral vector-mediated antiangiogenic gene transfer in malignant ascites. Hum Gene Ther. 2001;12:1713-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Nguyen JT. Adeno-associated virus and other potential vectors for angiostatin and endostatin gene therapy. Adv Exp Med Biol. 2000;465:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Nguyen JT, Wu P, Clouse ME, Hlatky L, Terwilliger EF. Adeno-associated virus-mediated delivery of antiangiogenic factors as an antitumor strategy. Cancer Res. 1998;58:5673-5677. [PubMed] |
| 15. | Cao GW, Qi ZT, Pan X, Zhang XQ, Miao XH, Feng Y, Lu XH, Kuriyama S, Du P. Gene therapy for human colorectal carcinoma using human CEA promoter contro led bacterial ADP-ribosylating toxin genes human CEA: PEA & amp; DTA gene transfer. World J Gastroenterol. 1998;4:388-391. [PubMed] |
| 16. | Li Z, Pan X, Pan W, Cao GS, Wen ZZ, Fang GE, Qi ZT, Bi JW, Hua JD. Packaging and identification of recombinant adenovi-rus carrying endostatin-soluble vascular endothelial growth inhibitor gene. Shijie Huaren Xiaohua Zazhi. 2003;11:741-744. |
| 17. | Pan X, Li Z, Zhang M, Wang Y, Pan W, Qi ZT. Therapeutic effect of endostatin-vascular endothelial growth inhibitor re-combinant adenoviruses on gastric carcinoma in nude mice. Shijie Huaren Xiaohua Zazhi. 2003;11:1282-1285. |
| 18. | Nyberg-Hoffman C, Shabram P, Li W, Giroux D, Aguilar-Cordova E. Sensitivity and reproducibility in adenoviral infectious titer determination. Nat Med. 1997;3:808-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Michie J, Akudugu J, Binder A, Van Rensburg CE, Böhm L. Flow cytometric evaluation of apoptosis and cell viability as a criterion of anti-tumour drug toxicity. Anticancer Res. 2003;23:2675-2679. [PubMed] |
| 20. | Zhao CS, Zhang L, Shen YP. A preventive and therapeutic study of experimental corneal neovascularization. Tongji Yike Daxue Xuebao. 1996;25:399-401. |
| 21. | Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA. 1998;95:14389-14394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 442] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 22. | Saita N, Fujiwara N, Yano I, Soejima K, Kobayashi K. Trehalose 6,6'-dimycolate (cord factor) of Mycobacterium tuberculosis induces corneal angiogenesis in rats. Infect Immun. 2000;68:5991-5997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Volpert OV, Fong T, Koch AE, Peterson JD, Waltenbaugh C, Tepper RI, Bouck NP. Inhibition of angiogenesis by interleukin 4. J Exp Med. 1998;188:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 176] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Seo K, Choi J, Park M, Rhee C. Angiogenesis effects of nerve growth factor (NGF) on rat corneas. J Vet Sci. 2001;2:125-130. [PubMed] |
| 26. | Chew LJ, Pan H, Yu J, Tian S, Huang WQ, Zhang JY, Pang S, Li LY. A novel secreted splice variant of vascular endothelial cell growth inhibitor. FASEB J. 2002;16:742-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Nör JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM, Polverini PJ. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61:2183-2188. [PubMed] |
| 28. | Olewniczak S, Chosia M, Kwas A, Kram A, Domagała W. Angiogenesis and some prognostic parameters of invasive ductal breast carcinoma in women. Pol J Pathol. 2002;53:183-188. [PubMed] |
| 29. | Wang L, Pan W, Zhu FL, Jiao BH, Lou YH, Xiao Y, Qi ZT. Cloning, Expression and Biological Activity of VEGI(151), a Novel Vascular Endothelial Cell Growth Inhibitor. Shengwu Huaxue Yu Shengwu Wuli Xuebao (Shanghai). 2000;32:485-489. [PubMed] |
| 30. | Cao MM, Pan W, Chen QL, Ma ZC, Ni ZJ, Wu XL, Wu WB, Pan X, Cao GW, Qi ZT. Construction of the eukaryotic expres-sion vector expressing the fusion protein of human endostatin protein and IL3 signal peptide. Shijie Huaren Xiaohua Zazhi. 2001;9:43-46. |
| 31. | O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3131] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 32. | Zhai Y, Yu J, Iruela-Arispe L, Huang WQ, Wang Z, Hayes AJ, Lu J, Jiang G, Rojas L, Lippman ME. Inhibition of angiogenesis and breast cancer xenograft tumor growth by VEGI, a novel cytokine of the TNF superfamily. Int J Cancer. 1999;82:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Dhanabal M, Volk R, Ramchandran R, Simons M, Sukhatme VP. Cloning, expression, and in vitro activity of human endostatin. Biochem Biophys Res Commun. 1999;258:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Ding I, Sun JZ, Fenton B, Liu WM, Kimsely P, Okunieff P, Min W. Intratumoral administration of endostatin plasmid inhibits vascular growth and perfusion in MCa-4 murine mammary carcinomas. Cancer Res. 2001;61:526-531. [PubMed] |
Edited by Wang XL and Xu FM