Published online Jan 1, 2004. doi: 10.3748/wjg.v10.i1.58
Revised: May 20, 2003
Accepted: June 12, 2003
Published online: January 1, 2004
AIM: To explore the feasibility of computed tomography (CT)-guided percutaneous ethanol injection (PEI) using a disposable curved needle for treatment of malignant liver neoplasms and their metastases in retroperitoneal lymph nodes.
METHODS: CT-guided PEI was conducted using a disposable curved needle in 26 malignant liver tumors smaller than 5 cm in diameter and 5 lymph node metastases of liver cancer in the retroperitoneal space. The disposable curved needle was composed of a straight trocar (21G) and stylet, a disposable curved tip (25 G) and a fine stylet. For the tumors found in deep sites and difficult to reach, or for hepatic masses inaccessible to the injection using a straight needle because of portal vein and bile ducts, the straight trocar was used at first to reach the side of the tumor. Then, the disposable curved needle was used via the trocar. When the needle reached the tumor center, appropriate amount of ethanol was injected. For relatively large malignant liver tumors, multi-point injection was carried out for a better distribution of the ethanol injected throughout the masses. The curved needle was also used for treatment of the metastasis in retroperitoneal lymph nodes blocked by blood vessels and inaccessible by the straight needle.
RESULTS: All of the 26 liver tumors received 2 or more times of successful PEI, through which ethanol was distributed throughout the whole tumor mass. Effect of the treatment was monitored by contrast-enhanced multi-phase CT and magnetic resonance imaging (MRI) examinations three months later. Of the 18 lesions whose diameters were smaller than 3 cm, the necrotic change across the whole mass and that in most areas were observed in 15 and 3 tumors, respectively. Among the 8 tumors sizing up to 5 cm, 5 were completely necrotic and 3 largely necrotic. Levels of tumor seromarkers were significantly reduced in some of the cases. In 5 patients with metastases of liver cancer in retroperitoneal lymph nodes who received 1 to 3 times of PEI, all the foci treated were completely necrotic and smaller demonstrated by dynamic contrast-enhanced CT or MRI 3 months later.
CONCLUSION: CT-guided PEI using a disposable curved needle is effective, time-saving and convenient, providing an alternative therapy for the treatment of malignant liver tumors and their retroperitoneal lymph node metastases.
- Citation: Zuo CJ, Wang PJ, Shao CW, Wang MJ, Tian JM, Xiao Y, Ren FY, Hao XY, Yuan M. CT-guided percutaneous ethanol injection with disposable curved needle for treatment of malignant liver neoplasms and their metastases in retroperitoneal lymph nodes. World J Gastroenterol 2004; 10(1): 58-61
- URL: https://www.wjgnet.com/1007-9327/full/v10/i1/58.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i1.58
Percutaneous ethanol injection (PEI) is a common procedure for the treatment of malignant liver neoplasms. But in the treatment of small or deep foci with CT-guided PEI, repeated punctures are necessary for the needle tip to reach an appropriate position. This may result in prolongation of the operation time and an increase of the risk for complications. This article reported our recent experience in PEI treatment of liver tumors using a disposable curved needle.
Thirty-one patients (22 males, 9 females; 37 to 72 years old, mean 48.9 years) were included in this study. Of them, 19 had primary liver cancer and 7 had metastatic liver tumors, including a single focus in 15 patients and multiple foci ( ≥ two tumors) in 11 patients. Twenty-six foci in 26 patients were treated by PEI using a disposable curved needle, including 18 smaller than 3 cm in diameter and 8 sizing from 3 cm to 5 cm. Metastases in retroperitoneal lymph nodes were found in 5 patients, with a single node involved in 3, 2 nodes in one and 3 nodes in the other. All of the five patients received PEI treatment, one lesion for each. Every patient was informed of possible complications and the consent was obtained before operation. The procedure was approved by the Ethics Commission of the hospital.
PQ 5000 V helical CT machine (Picker International INC, Cleveland, Ohio, USA) was used to guide the puncture. The disposable curved needle set (DCHNS, COOK, Bloomington, USA) is composed of a tip (25 G), a fine stylet, a straight trocar (21 G) and a trocar stylet. Volume Zoom multi-slice helical CT scanner (Siemens AG, Forchheim,Germany) and Symphony 1.5T MRI machine (Siemens AG, Munchen, Germany) were used to evaluate the post-operative lesions.
The location, morphology and size of the focus were determined by enhanced CT or MRI. Prothrombin time, blood routine, liver function and tumor markers were evaluated. The patients were fasted for four hours and notified of precautions before the procedure. Sedatives were administered for patients with mental stress.
An appropriate posture was selected and the skin surface was marked. The focal area was scanned by 5-10 mm thickness to determine the optimal puncture point, angle and depth. Local sterilization, draping and local anesthesia with 2% lidocaine were conducted. The patients were advised to hold breath at rest, when the needle was inserted into the focus according to the preplanned angle. The needle was adjusted under CT guidance in case of any deviation. The disposable curved needle was used in the following cases: 1) foci too small in size or too deep in location for the straight needle, 2) the way to the tumor center blocked by the portal vessel or bile dust, 3) large foci with multiple-site injections of ethanol by one puncture. When the straight trocar of the disposable curved needle reached the proximity of the tumor, stylet was withdrawn and the 25 G curved needle was inserted. The needle tip was 90º to the needle body in a natural state. When the tip traveled out of the trocar, it was bent by its own elasticity. The next step was to direct the tip to the focus and insert into it the tumor. Then, the stylet was removed and ethanol was injected. For the larger foci, the trocar was inserted, through which ethanol was injected from superficial to deeper areas. After that, the flexible needle was inserted, through which ethanol was injected into the areas around the trocar. Before injecting ethanol, a test withdrawal was done to make sure that the needle was not misled to the blood vessel or biliary duct. Ethanol injection should be sufficient to diffuse to all or most parts of the tumor. The total volume of ethanol injected each time should not exceed 40 mL. As absolute alcohol is of low density, it could be mixed with a small amount of contrast medium to increase its density so that the diffusion of ethanol could be more visible. A small amount of the anesthetic was administered while the needle was being withdrawn. When the needle was removed, a check-up was necessary to see whether the ethanol was well distributed within the tumor, whether there was ethanol reflex, or whether passage of the needle through the pulmonary region caused pneumothorax.
Postoperative treatment included fluid replacement, analgesic and liver protection therapies, and re-examination of liver function and tumor markers. A second treatment was given at an interval of 5 to 7 days.
Multi-slice helical CT scan or contrast-enhanced MRI was done three months after PEI to evaluate the outcome by the necrotic area of the foci, changes of the tumor marker level and clinical manifestations.
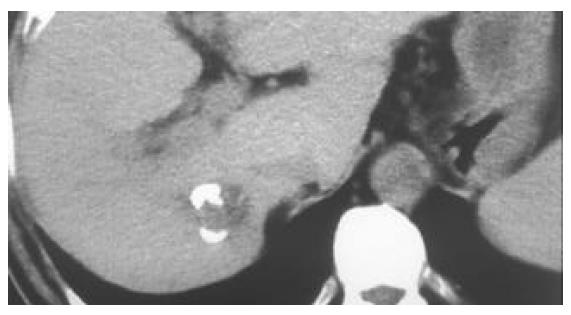
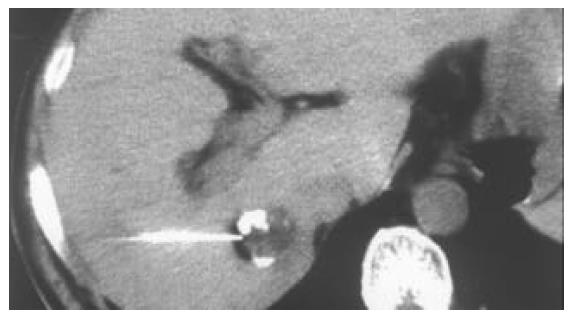
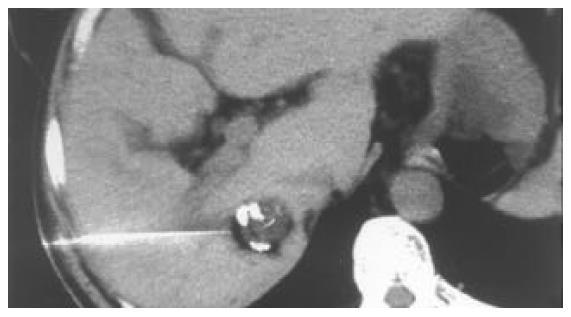
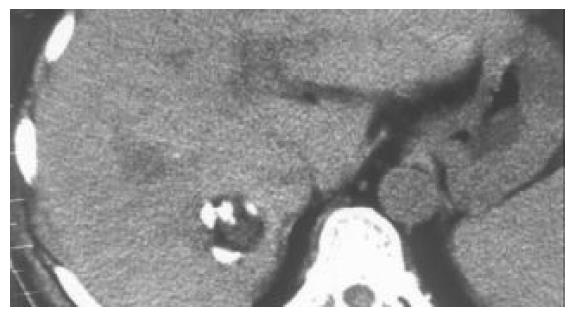
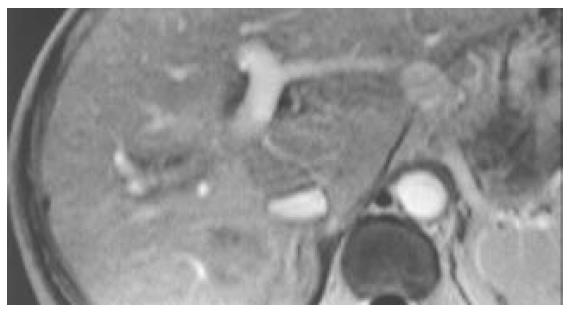
All of the 26 tumors received 2 or more times of CT-guided PEI. Single-point or multi-point injections were performed for foci smaller than 3 cm, and multi-point injections for all of the foci larger than 3 cm in diameter. The ethanol distribution was found to cover the whole tumor areas in all these 26 cases (Figures 1, Figures 2, Figures 3, Figures 4). CT or MRI images showed that, of the 18 foci smaller than 3 cm, 15 were completely necrotic (Figure 5) and the remaining three were mostly necrotic. Of the 8 foci larger than 3 cm, 5 were completely necrotic and the other three were mostly necrotic. In four patients who were found to have residue nodules, CT-guided PEI was performed again, and AFP level declined markedly and the symptoms improved in some of these patients. Apart from abdominal pain of varying degrees during the procedure, no other severe complication was observed.
The common treatments for malignant liver tumors include surgery, transcatheter arterial chemoembolization (TACE), PEI, radiofrequency thermal ablation, microwave coagulation, laser thermal ablation therapy[1-4]. Although surgery is the most radical treatment, it is not indicative for patients with severe cirrhosis or in cases where tumors are very close to the major vessels. Surgery was not usually preferable if there were multiple foci within the liver[5]. TACE is a good alternative, but it causes severe damage to the liver. The outcome of TACE is usually poor for tumors with insufficient blood supply. PEI is a better alternative approach for treatment of malignant liver tumors, as it has proved to be effective and to cause minimal trauma[6-10]. According to Ryu et al., PEI was the choice of treatment for clinical stage II patients with tumors smaller than 3 cm. For clinical stage I patients with such small tumors, selective PEI or surgical resection should be considered[11]. In these cases, the outcomes of PEI and surgery were similar but better than TACE. Therefore, PEI should be selected according to the conditions of individual patients[12]. Combination of PEI and TACE and other treatment was sometimes used to achieve optimal outcomes[13-18]. Moreover, PEI is more convenient and safer than radiofrequency thermal ablation and microwave coagulation therapy. PEI has become a preferable approach for lesions adjacent to main biliary ducts or to intestinal loops and for well-differentiated hepatocellular carcinoma[19-21].
Chemotherapy, radiotherapy and surgery have become the routine treatments for retroperitoneal metastases of liver cancer and other malignant tumors[22-28], but some of the lesions are not sensitive to radiotherapy and chemotherapy. In addition, radiotherapy and chemotherapy are more traumatic and expensive. Dissection of metastatic lymph nodes usually results in more traumas and complications. Some patients whose general conditions are poor can not tolerate chemotherapy, radiotherapy or surgery. While CT-guided PEI is more effective for the treatment of lymph node metastases in the retroperitoneal space and abdominal cavity with less trauma and lower cost. Following CT-guided PEI, appropriate chemotherapy and radiotherapy still can be considered.
Clear visualization of tumor location and ethanol distribution during CT-guided PEI for malignant liver tumors makes it possible to inject sufficient ethanol for a better outcome. It is especially suitable for foci that could be clearly detected on ultrasound[29]. For the CT-guided PEI, it is a key step to insert the needle accurately into the target. Using the needle with a disposable curved tip, it is convenient to reach the foci and to avoid disadvantages of routine PEI procedure. What you need to do is to insert the trocar to the edge of the tumor and the disposable curved needle tip into the tumor through the trocar. It saves time and reduces complications. The disposable curved tip can easily reach a deeply embedded tumor and avoid possible damages to the surrounding vessels or bile ducts. For tumors larger than 3 cm, multiple injections are necessary. By the procedure described here, a straight trocar is used at first to inject ethanol, and then the disposable curved needle tip is inserted through the trocar to inject ethanol into areas that the straight trocar cannot reach, so that ethanol can diffuse to the whole tumor. Retroperitoneal lymph nodes are usually deep and adjacent to abdominal aorta and inferior vena cava, PEI is usually done under CT guidance because CT guidance is accurate and the distribution of ethanol within the metastatic lymph node can be clearly visualized. The disposable curved needle can be used for metastatic lymph nodes in way to which is blocked by vessels. For larger metastatic lymph nodes, multiple-site injections of ethanol by one puncture can be done using a disposable curved needle and the procedure is more convenient than that using a routine straight needle.
As we know, this is the first report describing the use of a disposable curved puncture needle in CT-guided PEI for malignant liver tumors and retroperitoneal lymph node metastases. This procedure could also be used in CT-guided acetic acid injection for liver tumors[30,31], ethanol injection for adrenal tumors[32], and thymus ethanol injection for myasthenia gravis[33].
| 1. | Goldberg SN, Ahmed M. Minimally invasive image-guided therapies for hepatocellular carcinoma. J Clin Gastroenterol. 2002;35:S115-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Livraghi T. Radiofrequency ablation, PEIT, and TACE for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003;10:67-76. [PubMed] |
| 3. | Seki T, Tamai T, Nakagawa T, Imamura M, Nishimura A, Yamashiki N, Ikeda K, Inoue K. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. 2000;89:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Pacella CM, Bizzarri G, Cecconi P, Caspani B, Magnolfi F, Bianchini A, Anelli V, Pacella S, Rossi Z. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Livraghi T. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. Hepatogastroenterology. 2001;48:20-24. [PubMed] |
| 7. | Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S, Torzilli G. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 604] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Lin SM, Lin DY, Lin CJ. Percutaneous ethanol injection therapy in 47 cirrhotic patients with hepatocellular carcinoma 5 cm or less: a long-term result. Int J Clin Pract. 1999;53:257-262. [PubMed] |
| 9. | Livraghi T, Benedini V, Lazzaroni S, Meloni F, Torzilli G, Vettori C. Long term results of single session percutaneous ethanol injection in patients with large hepatocellular carcinoma. Cancer. 1998;83:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Takayasu K, Muramatsu Y, Asai S, Muramatsu Y, Kobayashi T. CT fluoroscopy-assisted needle puncture and ethanol injection for hepatocellular carcinoma: a preliminary study. AJR Am J Roentgenol. 1999;173:1219-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ryu M, Shimamura Y, Kinoshita T, Konishi M, Kawano N, Iwasaki M, Furuse J, Yoshino M, Moriyama N, Sugita M. Therapeutic results of resection, transcatheter arterial embolization and percutaneous transhepatic ethanol injection in 3225 patients with hepatocellular carcinoma: a retrospective multicenter study. Jpn J Clin Oncol. 1997;27:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Ueno S, Tanabe G, Nuruki K, Oketani M, Komorizono Y, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Prognosis of hepatocellular carcinoma associated with Child class B and C cirrhosis in relation to treatment: a multivariate analysis of 411 patients at a single center. J Hepatobiliary Pancreat Surg. 2002;9:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Tanaka K, Nakamura S, Numata K, Kondo M, Morita K, Kitamura T, Saito S, Kiba T, Okazaki H, Sekihara H. The long term efficacy of combined transcatheter arterial embolization and percutaneous ethanol injection in the treatment of patients with large hepatocellular carcinoma and cirrhosis. Cancer. 1998;82:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Kirchhoff T, Chavan A, Galanski M. Transarterial chemoembolization and percutaneous ethanol injection therapy in patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1998;10:907-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Lencioni R, Paolicchi A, Moretti M, Pinto F, Armillotta N, Di Giulio M, Cicorelli A, Donati F, Cioni D, Bartolozzi C. Combined transcatheter arterial chemoembolization and percutaneous ethanol injection for the treatment of large hepatocellular carcinoma: local therapeutic effect and long-term survival rate. Eur Radiol. 1998;8:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Koda M, Murawaki Y, Mitsuda A, Oyama K, Okamoto K, Idobe Y, Suou T, Kawasaki H. Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma: a randomized control study. Cancer. 2001;92:1516-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Kamada K, Kitamoto M, Aikata H, Kawakami Y, Kono H, Imamura M, Nakanishi T, Chayama K. Combination of transcatheter arterial chemoembolization using cisplatin-lipiodol suspension and percutaneous ethanol injection for treatment of advanced small hepatocellular carcinoma. Am J Surg. 2002;184:284-290. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Guo WJ, Yu EX, Liu LM, Li J, Chen Z, Lin JH, Meng ZQ, Feng Y. Comparison between chemoembolization combined with radiotherapy and chemoembolization alone for large hepatocellular carcinoma. World J Gastroenterol. 2003;9:1697-1701. [PubMed] |
| 19. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 878] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 20. | Livraghi T, Lazzaroni S, Meloni F. Radiofrequency thermal ablation of hepatocellular carcinoma. Eur J Ultrasound. 2001;13:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Horigome H, Nomura T, Saso K, Itoh M. Standards for selecting percutaneous ethanol injection therapy or percutaneous microwave coagulation therapy for solitary small hepatocellular carcinoma: consideration of local recurrence. Am J Gastroenterol. 1999;94:1914-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Uenishi T, Hirohashi K, Tanaka H, Yamamoto T, Kubo S, Kinoshita H. A surgically treated case of hepatocellular carcinoma with extensive lymph node metastases. Hepatogastroenterology. 2000;47:1714-1716. [PubMed] |
| 23. | Uenishi T, Hirohashi K, Shuto T, Kubo S, Tanaka H, Sakata C, Ikebe T, Kinoshita H. The clinical significance of lymph node metastases in patients undergoing surgery for hepatocellular carcinoma. Surg Today. 2000;30:892-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Mosharafa AA, Foster RS, Leibovich BC, Bihrle R, Johnson C, Donohue JP. Is post-chemotherapy resection of seminomatous elements associated with higher acute morbidity. J Urol. 2003;169:2126-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Foster R, Bihrle R. Current status of retroperitoneal lymph node dissection and testicular cancer: when to operate. Cancer Control. 2002;9:277-283. [PubMed] |
| 26. | Hendry WF, Norman AR, Dearnaley DP, Fisher C, Nicholls J, Huddart RA, Horwich A. Metastatic nonseminomatous germ cell tumors of the testis: results of elective and salvage surgery for patients with residual retroperitoneal masses. Cancer. 2002;94:1668-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Steyerberg EW, Marshall PB, Keizer HJ, Habbema JD. Resection of small, residual retroperitoneal masses after chemotherapy for nonseminomatous testicular cancer: a decision analysis. Cancer. 1999;85:1331-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Hermans BP, Foster RS, Bihrle R, Little S, Sandler A, Einhorn LH, Donohue JP. Is retroperitoneal lymph node dissection necessary for adult paratesticular rhabdomyosarcoma. J Urol. 1998;160:2074-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Lee MJ, Mueller PR, Dawson SL, Gazelle SG, Hahn PF, Goldberg MA, Boland GW. Percutaneous ethanol injection for the treatment of hepatic tumors: indications, mechanism of action, technique, and efficacy. AJR Am J Roentgenol. 1995;164:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Ohnishi K, Yoshioka H, Ito S, Fujiwara K. Prospective randomized controlled trial comparing percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatology. 1998;27:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Arrivé L, Rosmorduc O, Dahan H, Fartoux L, Monnier-Cholley L, Lewin M, Poupon R, Tubiana JM. Percutaneous acetic acid injection for hepatocellular carcinoma: using CT fluoroscopy to evaluate distribution of acetic acid mixed with an iodinated contrast agent. AJR Am J Roentgenol. 2003;180:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Wang P, Zuo C, Qian Z, Tian J, Ren F, Zhou D. Computerized tomography guided percutaneous ethanol injection for the treatment of hyperfunctioning pheochromocytoma. J Urol. 2003;170:1132-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Wang P, Zuo C, Tian J, Qian Z, Ren F, Shao C, Wang M, Lu T. CT-guided percutaneous ethanol injection of the thymus for treatment of myasthenia gravis. AJR Am J Roentgenol. 2003;181:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Edited by Su Q and Wang XL