INTRODUCTION
Extract from the leaves of Ginkgo biloba (maidenhair tree) has been used therapeutically in China and Western countries for centuries. The liquid extract was made from dried leaves with an extraction ratio of 1:50[1]. Standard Ginkgo bilobaextract, EGb 761 (commercial name), contains 22%-27% flavonoids (ginkgo-flavone glycosides) and 5%-7% terpenoids (ginkgolides and bilobalides), which are the most important active substances in the extract. The most important flavonoids are glycosides of kaempferol, quercetin, and isorhamnetin with glucose or rhamnose. Ginkgolides, not found in any other living species and only present in Ginkgo biloba extract, can be divided into types A, B, C, and a very small quantity of J, which are only different in the number and position of hydroxyl groups.
Ginkgo biloba extract has been mentioned in the traditional Chinese pharmacopoeia, and used for the treatment of asthma and bronchitis[1]. Ginkgo biloba extract is well known for its antioxidant property, which may result from its ability to scavenge free radicals[2], and to neutralize ferryl ion-induced peroxidation[3]. Several studies have reported that the antioxidant activity of Ginkgo biloba extract could be helpful in the prevention and therapy of diseases and degenerative processes associated with oxidative stress[4-8]. However, there have been very limited studies on the anti-proliferative activity of Ginkgo biloba extract. Ginkgetin or isoginkgetin, a biflavonoid from Ginkgo biloba extract, at 10 μmol/L showed a suppressive activity against lymphocyte proliferation induced by concanavalin A (ConA) or lipopolysaccharides (LPS)[9]. Additionally, Su et al.[10] found that ginkgetin selectively inhibited the proliferation of human ovarian carcinoma OVCAR-3 cells via the induction of apoptosis in a dose-dependent manner. So far, no study has investigated the effect of Ginkgo biloba extract on hepatocellular carcinoma (HCC), which is the most common malignant tumor occurred in men in Taiwan. It is reasonably hypothesized that Ginkgo biloba extract may be helpful for the therapy of HCC through regulating cell proliferation and/or cell death. Therefore, the purpose of this study was to investigate the cytotoxic effect of Ginkgo biloba extract on HCC.
MATERIALS AND METHODS
Cell culture and treatments
Human hepatocellular carcinoma cell lines, HepG2 (BCRC No. 60025) and Hep3B2.1-7 (Hep3B, BCRC No. 60434) were purchased from the Bioresources Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (Hsinchu, Taiwan). Cells were grown in 900 mL/L minimum essential medium (MEM, GIBCOTM, Invitrogen Corp., Carlsbad, CA) containing 100 mL/L fetal bovine serum (GIBCOTM) at 37 °C in a humidified atmosphere of 950 mL/L air and 50 mL/L CO2. Prior to addition of the treatment, the cells were grown to 80%-90% confluency and synchronized by incubating in the basal medium (100% MEM) for 24 h. The cells were then incubated with various concentrations (0-1000 mg/L) of standard Ginkgo biloba extract solution (EGb 761, Cerenin®, Dr. Willmar Schwabe GmbH and Co., Karlsruhe, Germany) for 24 or 48 h. Ginkgo biloba extract contained 22%-27% flavonoids and 5%-7% terpenoids in 10 mL/L ethanol and 10 mL/L sorbitol solution. Cells without addition of Ginkgo biloba extract as the control group were incubated with 10 mL/L ethanol and 10 mL/L sorbitol solution. The cells and medium were collected. Protein contents in cells and medium were measured by the modified method of Lowry et al.[11] using a Bio-Rad DC protein kit (Bio-Rad Laboratories, Hercules, CA).
Cell proliferation assay
Cell viability was colorimetrically determined at 490 nm using a commercial proliferation assay kit (CellTiter 96® AQueous, Promega Corp., Madison, WI). After incubation with various concentrations of EGb 761 for 24 h, the cells (n = 8) in a 96-well plate were incubated with 333 mg/L 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and 25 μmol/L phenazine methosulfate solution for 3 h at 37 °C in a humidified, 50 mL/L CO2 atmosphere[12]. The absorbance of soluble formazan produced by cellular reduction of MTS was measured at 490 nm using an ELISA reader (Multiskan RC, Labsystems, Helsinki, Finland).
Cell cytotoxicity assay
Lactate dehydrogenase (LDH) release from cells was determined as an index of cytotoxicity or necrosis. The quantity of LDH released by the cells into the medium was measured by the decrease in the absorbance at 340 nm for NADH disappearance within 5 min[13]. After incubation with various concentrations of EGb 761 for 24 h, the cell culture supernatant and medium (100 μL) were mixed with 900 μL of modified Krebs-Henseleit buffer (118 mmol/L NaCl, 4.8 mmol/L KCl, 1 mmol/L KH2PO4, 24 mmol/L NaHCO3, 3 mmol/L CaCl2, 0.8 mmol/L MgPO4, pH 7.4), 0.2 mmol/L NADH, 1.36 mmol/L sodium pyruvate, and 20 mg/L bovine serum albumin (BSA). Percentage of LDH release (n = 3) was equal to LDH activity in medium divided by that in both cell culture supernatant and medium × 100%.
Analysis of proliferating cell nuclear antigen (PCNA) and p53 protein
After incubation with various concentrations of EGb 761 for 48 h, cell suspension (15 μg and 50 μg protein for PCNA and p53) pooled from 6 independent experiments (n = 6) was mixed with an equal volume of 2 × SDS-PAGE sample buffer (0.125 mol/L Tris-HCl, pH 6.8/40 mg/L SDS/200 mL/L glycerol/100 mL/L 2-mercaptoethanol)[14], denatured at 100 °C for 3 min, and applied to SDS-PAGE (Bio-Rad Mini-PROTEAN 3 Cell, Bio-Rad Laboratories). Proteins were separated by 12.5% resolving gel, with 4% stacking gel in the running buffer (25 mmol/L Tris, pH 8.3/192 mmol/L glycine/ 1 mg/L SDS) at 100 V for 1.5 h. After that, the proteins were transferred onto nitrocellulose membrane (0.45 μm) using a semi-dry transfer unit (Hoefer Semiphor TE 70, Amersham Biosciences Corp., San Francisco, CA) in Towbin buffer (25 mmol/L Tris/192 mmol/L glycine/1.3 mmol/L SDS/100 mL/L methanol) at 200 mA for 1.5 h[15]. The membrane was washed briefly with PBS, and incubated with a blocking buffer (50 mg/L skim milk/1 mL/L Tween-20 in PBS) for 1 h. Then the membrane was incubated with 0.4 mg/L mouse anti-human PCNA (PC-10, 0.2 mg/L), p53 (DO-1, 0.4 mg/L), or α-tubulin (TU-02, 1 mg/L) mAb (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at room temperature for 1 h. Alpha-tubulin was as an internal control. The membrane was washed 3 times with the wash buffer (1 mL/L Tween-20 in PBS), and incubated with 0.2 mg/L goat anti-mouse IgG-horseradish peroxide conjugate (Santa Cruz Biotechnology, Inc.) for 1.5 h. The blot was washed 3 times again with the wash buffer, incubated with luminol reagent (Santa Cruz Biotechnology, Inc.) for 1 min, and exposed to an X-ray film (Eastman Kodak Co., Rochester, NY) for 5-10 s. The bands were quantitated by an image analysis system (Gel analysis system, EverGene Biotechnology, Taipei, Taiwan) and Phoretix 1D Lite software (version 4.0, Phoretix International Ltd., Newcastle upon Tyne, UK).
Statistical analysis
Data are expressed as (-χ±s. Data were analyzed by one-way ANOVA to determine the treatment effect using SAS (version 8.2, SAS Institute Inc., Cary, NC). Fisher’s least significant difference test was used to make post-hoc comparisons if the treatment effect was demonstrated. Differences were considered significant when P < 0.05.
RESULTS
Cell proliferation assay
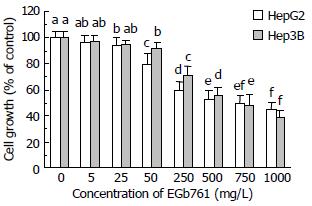
After 24 h incubation, EGb 761 (25-1000 mg/L) significantly inhibited cell growth (P < 0.05) in HepG2 cells compared with the control group in a dose-dependent manner determined by MTS assay (Figure 1). EGb 761 at 750 and 1000 mg/L inhibited cell growth to 50% and 45% of the control, respectively, in HepG2 cells. Similarly, EGb 761 (50-1000 mg/L) dose-dependently inhibited cell growth (P < 0.05) in Hep3B cells compared with the control group. EGb 761 at 750 and 1000 mg/L inhibited cell growth to 48% and 39% of the control, respectively, in Hep3B cells.
Figure 1 Effects of EGb 761 on cell growth in HepG2 and Hep3B cells measured by MTS assay.
Data are expressed as -χ±s (n = 8). Values not sharing the same letter differed significantly (P < 0.05) in the same cell line by Fisher’s least significant difference test.
Cell cytotoxicity assay
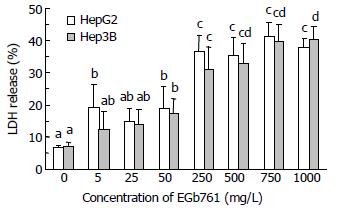
Cell cytotoxicity was directly measured by LDH release. After 24 h incubation, EGb 761 (50-1000 mg/L) significantly increased cell cytotoxicity (P < 0.05) in both HepG2 and Hep3B cells compared with the control group (6.7% and 7.2% in HepG2 and Hep3B cells) (Figure 2). EGb 761 at a dose of 250 mg/L significantly increased LDH release to 36.6% and 31.2% (P < 0.05), respectively, in HepG2 and Hep3B cells compared with EGb 761 at a dose of 50 mg/L (18.9% and 17.5% in HepG2 and Hep3B cells). However, EGb 761 at the dose of 250-1000 mg/L did not dose-dependently increase cell cytotoxicity in both HepG2 (35.2%-41.3% LDH release) and Hep3B (31.2%-40.3% LDH release) cells.
Figure 2 Effects of EGb 761 on cell cytotoxicity in HepG2 and Hep3B cells determined by lactate dehydrogenase (LDH) release.
Data are expressed as -χ±s (n = 3). Values not sharing the same letter differed significantly (P < 0.05) in the same cell line by Fisher’s least significant difference test.
Analysis of proliferating cell nuclear antigen (PCNA) and p53 protein
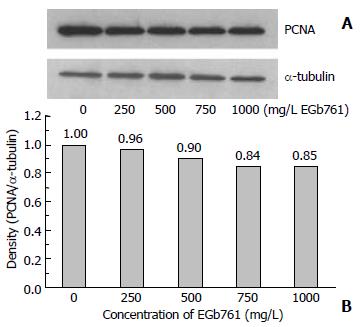
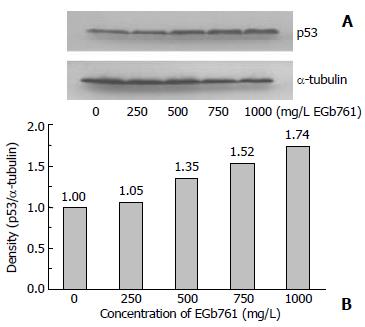
After 48 h incubation with EGb 761, the expression of PCNA and p53 protein in HepG2 cells was analyzed by SDS-PAGE and Western blotting. EGb 761 at the dose of 500, 750, and 1000 mg/L decreased PCNA expression to 90%, 84%, and 85% of the control, respectively (Figure 3). However, EGb 761 at the dose of 500, 750, and 1000 mg/L increased p53 expression to 135%, 152%, and 174% of the control, respectively (Figure 4).
Figure 3 Expression of proliferating cell nuclear antigen (PCNA) with the molecular weight of 36 kDa which was visu-alized by Western blotting (A) and quantitated by an image analysis system (B) after HepG2 cells were incubated with 0-1000 mg/L EGb 761 for 48 h.
Samples were pooled from 6 independent experiments (n = 6). Alpha-tubulin (55 kDa) was as an internal control.
Figure 4 Expression of p53 protein with the molecular weight of 53 kDa which was visualized by Western blotting (A) and quantitated by an image analysis system (B) after HepG2 cells were incubated with 0-1000 mg/L EGb 761 for 48 h.
Samples were pooled from 6 independent experiments (n = 6). Alpha-tubulin (55 kDa) was as an internal control.
DISCUSSION
Ginkgo biloba extract (EGb 761) at the dose of 50-1000 mg/L significantly decreased cell proliferation measured by MTS assay in a dose-dependent manner in both HepG2 and Hep3B cells with 50% inhibition at approximately 750 mg/L. Similarly, EGb 761 and ginkgolide B were found to inhibit cell proliferation in highly aggressive human breast cancer MDA-231 cells in a dose-dependent manner[16]. Another Ginkgo biloba extract, IPS200 devoid of proanthocyanidins, at 200 mg/L decreased cell proliferation of Hep3B cells[17]. EGb 761 also showed an inhibitory effect on cell proliferation of cultured vascular smooth muscle cells in a dose-dependent pattern in vitro and significantly decreased the percentage of proliferating cells in the balloon-injured abdominal aorta of cholesterol-fed rabbits in vivo[18]. Additionally, EGb 761 given orally at 50 or 100 mg/kg·d or superoxide dismutase injected intravenously at 15000 U/kg·d inhibited preretinal proliferation in pigmented rabbits, suggesting that antioxidants might efficiently prevent preretinal proliferation[19]. The biflavonoids of Ginkgo biloba extract, ginkgetin and isoginkgetin at 10 μmol/L, inhibited lymphocyte proliferation induced by Con A or LPS[9]. Ginkgetin was also found to dose-dependently inhibit the growth of human ovarian adenocarcinoma OVCAR-3 cells with 50% inhibition at 1.8 mg/L[20]. Quercetin, an important flavonoid of Ginkgo biloba extract, significantly inhibited cell proliferation of HCC cell lines BEL-7402, HuH-7, and HLE with a peak inhibition at 50 μmol/L[21]. The inhibitory effect of EGb 761 on cell proliferation may be attributed to its antioxidation capacity, however, other constituents of EGb 761 may contribute to its cytotoxic action. Recently, Ginkgo biloba extract has been reported to affect gene expression related to cell growth[17]. EGb 761 dramatically reduced, in a consistent manner, the expression of α-fetoprotein in Hep3B cells using fluorescence and membrane DNA microarrays, in which protein is present at very low concentration in adults but is increased in hepatoma patients. Moreover, IPS200 inhibited 40%-50% mRNA levels of peripheral-type benzodiazepine receptor, positively correlated with cell proliferation and metastasis in MDA-231, Hep3B, and U87 (human glioblastoma) cells using quantitative RT-PCR and Northern blot analysis. In contrast to our study, a previous study demonstrated that the components of Ginkgo biloba extract, such as quercetin, kaempferol, sciadopitysin, ginkgetin, and isoginkgetin, enhanced cell proliferation of normal human skin fibroblasts in vitro[22]. The various effects of Ginkgo biloba extract on cell proliferation may result from different cell lines, cell morphology (normal vs malignant), and the dosage of Ginkgo biloba extract or the individual constituents.
Besides the inhibitory effect of EGb 761 on cell proliferation, EGb 761 at higher doses (50-1000 mg/L) significantly increased cytotoxicity determined by LDH release in both HepG2 and Hep3B cells. However, the cytotoxic effect of EGb 761 reached the plateau at the dose over 250 mg/L. The cytotoxic effect of EGb 761 could be attributed to necrosis and/or apoptosis. Our result revealed increased LDH release as an index of necrosis in EGb 761-treated cells, indicating that EGb 761 could enhance necrosis. Although we did not directly measure apoptosis, the expression of p53 protein, as an inducer of apoptosis in human HCC cells[23], was increased in EGb 761-treated groups. There may exist the possibility that apoptosis contributes to the cytotoxic effect of EGb 761. The cytotoxic activity of EGb 761 has been suspected to derive from ginkgolic acids[24,25] and ginkgetin[10] through their induction of apoptosis. Ginkgolic acids restricted in EGb 761 at less than 5 μg/g have been recognized as hazardous compounds with suspected cytotoxic, allergenic, mutagenic, and carcinogenic properties[24,25]. Ginkgolic acids caused death of cultured chick embryonic neurons in a dose-dependent manner, and ginkgolic acid-induced death showed both signs of apoptosis and necrosis possibly via the mediation of protein phosphatase 2C[24]. Additionally, increased apoptotic cells from 6% (control) to nearly 80% were found in human keratinocyte cell line HaCaT incubated with ginkgolic acids at the concentration of more than 30 mg/L, which was primarily regulated by transformation of mitochondria probably induced by uncoupling of oxidative phosphorylation[25]. Ginkgetin (5 mg/L) increased intracellular levels of hydrogen peroxide and induced apoptosis in OVCAR-3 cells, which was mediated mainly through the activation of caspase (s) by the generation of hydrogen peroxide perhaps through autooxidation of ginkgetin[10]. However, some constituents of EGb 761 have been reported to possess anti-apoptotic capacity[26,27]. EGb 761 (100 mg/L), ginkgolide J (100 μmol/L), and ginkgolide B (10 μmol/L) reduced apoptosis from 74% in staurosporine-induced apoptotic chick embryonic neurons to 24%, 62%, and 31%, respectively[26]. Furthermore, EGb 761 (100 mg/L) and bilobalide (100 μmol/L) rescued rat neurons from apoptosis induced by serum deprivation. A previous study also found that the total flavonoids of EGb 761, two pure flavonoid components (rutin and quercetin), and a mixture of flavonoids and terpenes protected cerebellar granule cells from oxidative stress and apoptosis induced by hydroxyl radicals[27]. However, total terpenes of EGb 761 neither protected against apoptosis nor had a synergistic effect, suggesting that terpenes did not scavenge hydroxyl radicals directly to suppress apoptosis. The apoptosis-stimulatory and anti-apoptotic properties of EGb 761 might depend on the target cells, the dosage, and its constituents.
Both cell proliferation and apoptosis were involved in HCC[27]. The expression of PCNA and p53 proteins has been regarded as cell proliferation and apoptosis biomarkers, respectively, for the malignant phenotype of HCC, and was associated with prognosis and therapeutic outcome[28-31]. Cell proliferation was increased as HCC progressed to become more poorly differentiated[29,30]. Apoptotic HCC cancer cells were consistently negative for PCNA, and p53-positive cancer cells showed apoptosis[28]. Consistent with the result of MTS assay that EGb 761 suppressed cell proliferation of HCC cells, EGb 761 at the dose of 500-1000 mg/L decreased the expression of PCNA by 10%-16%. Additionally, the expression of p53 protein, measured in HepG2 cells expressing endogenous p53, while not in Hep3B cells lacking endogenous p53 expression[32], was increased by 35%-74% in EGb 761-treated groups (500-1000 mg/L). It suggested that EGb 761 might be potentially useful for the prevention of HCC progression. Ginkgo biloba extract or its constituent has been considered as an anti-cancer agent. A previous study showed that EGb 761 ameliorated the deleterious effects on the forestomach and liver in mice with benzo(a)pyrene-induced gastric carcinoma[33]. Furthermore, combined quercetin and a recombinant adenovirus vector expressing human p53, granulocyte-macrophage colony-stimulating factor (GM-CSF), and B7-1 (CD80) genes, as a combined anti-cancer agent, synergetically inhibited cell proliferation and induced apoptosis of HCC cells[21].
In conclusion, Ginkgo biloba extract at the concentration above 50 mg/L can significantly suppress cell proliferation and increase cell cytotoxicity in human hepatocellular carcinoma cell lines. Additionally, Ginkgo biloba extract can decrease PCNA and increase p53 protein expression in HepG2 cells, suggesting that Ginkgo biloba extract may regulate not only cell proliferation but also apoptosis of HCC cells. Therefore, Ginkgo biloba extract may have therapeutic potential for HCC.