Published online Oct 1, 1995. doi: 10.3748/wjg.v1.i1.9
Revised: June 25, 1995
Accepted: August 20, 1995
Published online: October 1, 1995
AIM: To analyze the relationship between intestinal metaplasia (IM) and gastric cancer (GC).
METHODS: The expression of the Span-1 and Ypan-1 antigens in GC (n = 110) and IM (n = 343) specimens was examined using the ABC immunohistochemical technique.
RESULTS: The expression rates of Span-1 and Ypan-1 in well and moderately differentiated adenocarcinoma (85.4% and 70.0%, respectively), signet-ring cell carcinoma (80.0%, 88.7%) and mucinous adenocarcinoma (88.6%, 76.5%) were significantly higher than the rates in poorly differentiated adenocarcinoma (48.6%, 45.9%), whereas the difference between early GC (59.2%, 65.4%) and advanced GC (73.8%, 65.5%) was insignificant. For IM, the expression of Span-1 was significantly higher in dysplasia, IM with GC, and chronic atrophic gastritis than in chronic superficial gastritis. In contrast, the expression of Ypan-1 was significantly higher only in IM with dysplasia (65.5%) than in chronic superficial gastritis (39.3%). When IM was classified into types I, II and III, the expression of both antigens in type III (79.0%, 75.2%) was higher than in type I (42.3%, 45.5%) and type II (51.2%, 50.0%), which themselves were similar.
CONCLUSION: Span-1 and Ypan-1 may be of value in detecting GC, even in the early stage, and type III IM should be considered precancerous.
- Citation: Fang DC, Liu WW. Expression of Span-21 and Ypan-21 in gastric cancer and subtypes of intestinal metaplasia. World J Gastroenterol 1995; 1(1): 9-12
- URL: https://www.wjgnet.com/1007-9327/full/v1/i1/9.htm
- DOI: https://dx.doi.org/10.3748/wjg.v1.i1.9
The relationship between intestinal metaplasia (IM) and gastric cancer (GC) is still an open question. Some[1,2] but not all[3] authors consider type III IM to be precancerous, as the columnar cells contain sulfomucin. We have previously shown that both GC and type III IM could be significantly stained by MG7, a monoclonal antibody highly specific for GC, which might prove to be a useful tumor marker for GC and its precursor cells[4]. Recently, two new tumor markers for pancreatic cancer, Span-1 and Ypan-1, have been found to be expressed in GC as well[5,6]. It is worthwhile to assess the expression of both Span-1 and Ypan-1 in GC and different types of IM so as to evaluate the usefulness of these monoclonal antibodies in the early diagnosis of GC and to clarify the relationship between IM and GC.
Specimens of GC (n = 110, including 26 early and 84 advanced) were obtained from surgically resected specimens. The specimens contained well and moderately differentiated adenocarcinoma (WMDAC, n = 41, including papillary AC and tubular AC), poorly differentiated adenocarcinoma (PDAC, n = 37), signet-ring cell carcinoma (SRCC, n = 15) and mucinous adenocarcinoma (MAC, n = 17). Specimens of IM (n = 343) were obtained through endoscopical biopsy from the antrum (n = 292), body (n = 38) and fundus (n = 13) of the stomach. The specimens were divided into 5 groups according to their accompanying lesions: dysplasia (n = 32), GC (n = 79, within 2 cm from the margin of cancer), chronic atrophic gastritis (CAG, n = 173), gastric ulcer (GU, n = 31) and chronic superficial gastritis (CSG, n = 28). All the IM specimens were classified as mild (n = 107), moderate (n = 152) and severe (n = 84) according to the criteria defined by The National Cooperative Research Group for GC in 1980. Specimens of apparently normal gastric mucosa (n = 20, obtained through biopsy), intestinal mucosa (n = 15, obtained from resected specimens) and colonic mucosa (n = 20, obtained through biopsy) were included in the study. Specimens of fetal gastric (n = 10), intestinal (n = 10) and colonic (n = 10) mucosa obtained from 16-24-week-old fetuses after water bag absorption were also included.
Murine monoclonal antibodies specific for Span-1 and Ypan-1, obtained from Kim (GI research Lab, Veterans Administration Medical Center, University of California), were produced against the human pancreatic cancer cell lines SW 1990 and Capan-2, respectively. The Vectastain ABC kit and biotinylated anti-mouse IgG were from Vector Laboratories, Inc. Reagents for histochemical mucin staining, including Alcian blue pH 2-5/PAS to visualize neutral and acid mucin, and high iron diamine to identify sulfomucin, were provided by The 3rd Shanghai Reagent Factory.
Specimens were fixed in 10% formalin and embedded in paraffin. Six serial 5 μm-thick sections of each specimen were prepared: one for hematoxylin/eosin (HE) staining for histological diagnosis, two for mucin staining, and three for ABC immunohistochemical staining. IM specimens were stained with AB pH 2-5/PAS to visualize neutral mucin and sialomucin, and a high iron diamine/AB pH 2-5 stain was used to detect sulfomucin and sialomucin. IM specimens were classified into three types according to the results of the mucin and HE stains, as suggested by Filipe[7]: Type I (complete): Characterized by mature absorptive and goblet cells, the latter secreting sialomucin; Paneth cells were often present. Type II (incomplete): Absorptive cells were few or absent; Columnar “intermediate” cells were present in various stages of differentiation, secreting neutral and sialomucin; Goblet cells were secreting mainly sialomucin and occasionally sulfomucin; Paneth cells might not appear. Type III (incomplete): Cell de-differentiation was more obvious than in type II IM specimens; “intermediate cells” were secreting predominantly sulfomucin; Goblet cells were secreting sulfomucin or sialomucin; Paneth cells were hardly seen. There was a variable degree of disorganized glandular architecture frequently present in type II and III IM specimens.
Span-1 and Ypan-1 mAbs were used at a dilution of 1:40, and the secondary antibody (biotinylated horse anti-mouse IgG) was used at a dilution of 1:100. A section of well-differentiated cancer known to be strongly positive for Span-1 and Ypan-1 was included in each assay as a positive control. As a negative control, the mAbs were replaced by phosphate buffer; none of the negative control sections showed staining.
Chi Square contingency tables were used for statistical analysis, and Fisher’s exact test was used for small numbers. A P value < 0.05 was considered significant.
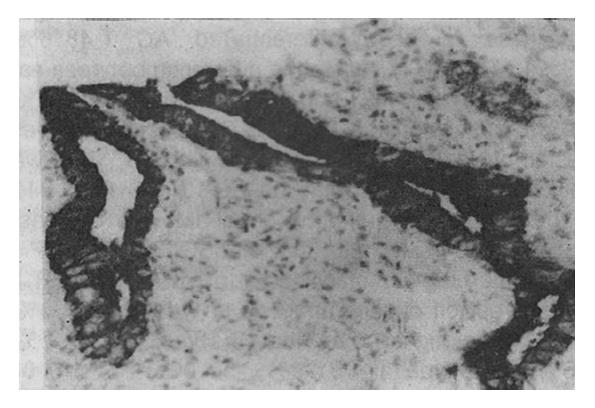
Eighty (72.7%) and 72 (65.5%) of 110 specimens of GC were positively stained for Span-1 and Ypan-1, respectively. The staining characteristics of both antigens in GC cells were essentially identical, appearing diffusely distributed in the cytoplasm. In some well-differentiated adenocarcinoma cells the antigenic material was found over the luminal surface of, or inside, the glandular cavities (Figure 1). The positive rates of Span-1 and Ypan-1 were 69.2% (18/26) and 65.4% (17/26), respectively, in the early GC specimens and 73.8% (62/84) and 65.5% (55/84) in the advanced GC specimens. The expression rates of both antigens among different types of GC are shown in Table 1.
| Histological type | n | Span-1 (%) | Ypan-1 (%) |
| WMDAC | 41 | 35 (85.4) | 29 (70.7) |
| PDAC | 37 | 12 (48.6)b | 17 (45.9) |
| SRCC | 15 | 12 (80.0) | 13 (86.7) |
| MAC | 17 | 15 (88.2) | 13 (76.5) |
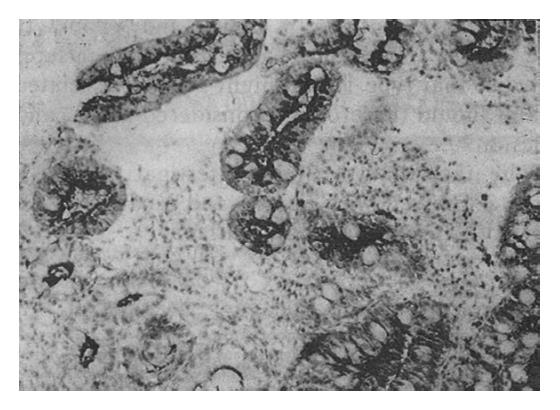
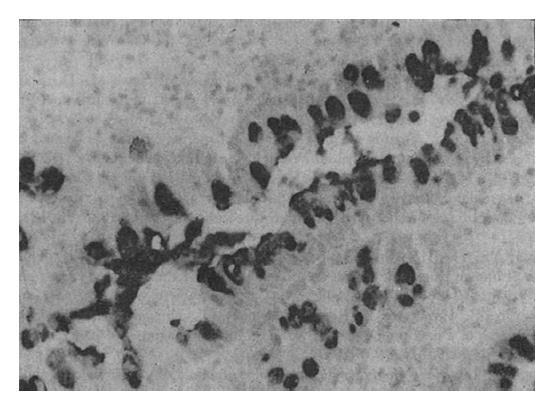
The Span-1 antibody stained the apical aspect of the epithelial cells; the basal aspect was usually not stained (Figure 2). Ypan-1 staining was found mainly in the mucus of goblet cells (Figure 3). The staining rates are shown in Table 2. The expression rate of Span-1 in the IM with CSG specimens was significantly lower than the rate in the dysplasia specimens and the IM with CAG specimens. The differences in Span-1 staining among the dysplasia, IM with GC, IM with CAG and IM with GU specimens were not significant. The only significant difference in Ypan-1 staining was a higher rate in dysplasia than in IM with CSG.
When the IM specimens were classified into types I (n = 156), II (n = 82) and III (n = 105), based on mucin and histological parameters, the expression rates of both antigens in type III IM were significantly higher than the rates in types I and II. The expression rates of both Span-1 and Ypan-1 were similar between IM types I and II (Table 3).
The expression of both Span-1 and Ypan-1 in different grades of IM is shown in Table 4. There were significant differences in Span-1 expression between the mild, moderate and severe grades of IM. In addition, the Ypan-1 expression rates in severe and moderate IM were significantly higher than the rate in mild IM.
The expression of both Span-1 and Ypan-1 in normal adult and fetal gastric, intestinal and colonic mucosa was essentially negative or occasionally weak, except for fetal intestinal mucosa which was strongly stained (90.0%, 60.0%) for both antigens (Table 5).
| Mucosa | n | Span-1 (%) | Ypan-1 (%) |
| Normal adult | |||
| Gastric | 20 | 0 | 0 |
| Intestinal | 15 | 0 | 1 (6.7) |
| Colonic | 20 | 0 | 4 (20.0) |
| Fetal | |||
| Gastric | 10 | 0 | 0 |
| Intestinal | 10 | 6 (60.0) | 9 (90.0) |
| Colonic | 10 | 0 | 2 (20.0) |
Span-1 is a high molecular weight glycoprotein recognized by a mAb produced against the human pancreatic cell line SW 1990[8]. Preliminary studies revealed a high sensitivity (81.3%-90.0%) of the mAb for human pancreatic cancers[9]. Recent immunohistochemical studies using the Span-1 mAb showed 89%, 67% and 62% positive staining of cancer specimens from the pancreas, stomach and colon, respectively[5]. Similar to Span-1, Ypan-1 is recognized by a murine mAb produced against the human pancreatic cancer cell line Capan-2. The Ypan-1 mAb showed immunoreactivity with formalin-fixed specimens of pancreatic (89%), gastric (90%), and colonic (46%) cancers[6]. However, a comprehensive survey of the Span-1 and Ypan-1 antigens in GC and its precursors has not been performed.
Our data firmly showed that both Span-1 and Ypan-1 are highly expressed in the different types of GC, except for PDAC which had a significantly lower expression rate. Both SRCC and MAC expressed the antigens to the same extent as WMDAC.
It is interesting that early GC lesions expressed as much Span-1 and Ypan-1 as did the advanced lesions, and that a considerable percentage of premalignant lesions such as dysplasia and IM also strongly expressed the antigens. Normal adult gastric mucosa was entirely negative for the immunoreactivity of both antigens. These facts lead us to believe that both Span-1 and Ypan-1 might be useful for the detection of early lesions of GC and its precursors.
The relationship between IM and GC has not been fully explored. Our previous work has repeatedly shown that IM with abundant sulfomucin in the columnar cells (type III) shares many biological characteristics with GC and thus should be treated as a precancerous lesion. In the present study, we found that, like dysplasia, those IMs which were adjacent to GC or accompanied with CAG were considerably immunoreactive for the Span-1 mAb. Moreover, type III IM expressed significantly more Span-1 and Ypan-1 than did IM types I and II. These data further support the possibility that type III IM is closely related to GC and should therefore be considered as a precancerous lesion.
The fact that fetal intestinal mucosa strongly expresses Span-1 and Ypan-1 indicates the oncofetal nature of both antigens.
| 1. | Jass JR, Filipe MI. The mucin profiles of normal gastric mucosa, intestinal metaplasia and its variants and gastric carcinoma. Histochem J. 1981;13:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Ng IOL, Ho J, Ng M. Intestinal metaplasia and gastric carcinoma: A histological study of 198 gastrectomy specimens. J Gastroenterol Hepatol. 1988;3:239-244. [DOI] [Full Text] |
| 3. | Ectors N, Dixon MF. The prognostic value of sulphomucin positive intestinal metaplasia in the development of gastric cancer. Histopathology. 1986;10:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Liu WW, Fang DC. Intestinal metaplasia and gastric cancer: An immunohistochemical and endoscopical followa2up study, Abstract I, The World Congress of. Gastroenterology Sydney, Australia. 1990;134. |
| 5. | Chung YS, Ho JJL, Satake K, Tanaka H, Nakata B, Sowa M. Distribution of Spana21 antigen in normal and cancerous human gastrointestinal tissues. Gastroenterology. 1988;94:A71. |
| 6. | Yuan SZ, Ho JJ, Yuan M, Kim YS. Human pancreatic cancer-associated antigens detected by murine monoclonal antibodies. Cancer Res. 1985;45:6179-6187. [PubMed] |
| 7. | Filipe MI, Potet F, Bogomoletz WV, Dawson PA, Fabiani B, Chauveinc P, Fenzy A, Gazzard B, Goldfain D, Zeegen R. Incomplete sulphomucin-secreting intestinal metaplasia for gastric cancer. Preliminary data from a prospective study from three centres. Gut. 1985;26:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Chung YS, Ho JJ, Kim YS, Tanaka H, Nakata B, Hiura A, Motoyoshi H, Satake K, Umeyama K. The detection of human pancreatic cancer-associated antigen in the serum of cancer patients. Cancer. 1987;60:1636-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Kiriyama S, Hayakawa T, Kondo T, Shibata T, Kitagawa M, Ono H, Sakai Y. Usefulness of a new tumor marker, Span-1, for the diagnosis of pancreatic cancer. Cancer. 1990;65:1557-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Presented at The
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Zhang FF