Published online Oct 1, 1995. doi: 10.3748/wjg.v1.i1.33
Revised: September 18, 1995
Accepted: September 26, 1995
Published online: October 1, 1995
AIM: To determine the serum concentration of alpha-fetoprotein (AFP) with anti-human AFP variant monoclonal antibody (AFP-R-LCA mAb) in detection of hepatocellular carcinoma (HCC) and to ascertain the value of this tumor marker in the diagnosis of HCC.
METHODS: Cell fusion was used for the preparation of anti-human AFP-R-LCA mAb which was assayed with a two-site sandwich enzyme linked immunosorbent assay (ELISA) method. Using this method, the serum concentration of AFP-R-LCA was tested in 99 patients with HCC, 67 patients with benign liver diseases (BLD), 30 pregnant women, and 30 normal controls.
RESULTS: The threshold value of the serum AFP-R-LCA was set to 10 μg/L, in reference to normal controls. The concentration of AFP-R-LCA in serum was 1.27 ± 0.6 μg/L and 0 μg/L in pregnant women and controls, respectively. As shown by the rocket immune-electrophoresis, the serum AFP-R-LCA increased from 62.6% to 86.8% in HCC patients (P < 0.05) while the positive rates were decreased from 19.4% to 1.45% in BLD patients (P < 0.05) and from 26.7% to 0% in pregnant women (P < 0.05). The false positive rate was 1.5%; the two-site sandwich ELISA assay had a specificity of 99.0% in the diagnosis of HCC.
CONCLUSION: Our AFP-R-LCA variant mAb had a higher affinity and specificity to AFP-R-LCA than the routinely used anti-AFP polyclonal antibody. Anti-human AFP-R-LCA variant mAb two-site sandwich ELISA assay had a low false positive rate so that it could be used for both early diagnosis of HCC and the differential diagnosis of HCC and BLD. The method is simple, accurate, and reproducible.
- Citation: Zhang BH, Wu MC. Evaluation of the serum alpha-fetoprotein-reactive-lentil-lectin in the diagnosis of hepatocellular carcinoma. World J Gastroenterol 1995; 1(1): 33-36
- URL: https://www.wjgnet.com/1007-9327/full/v1/i1/33.htm
- DOI: https://dx.doi.org/10.3748/wjg.v1.i1.33
Alpha-fetoprotein (AFP) is one of the most useful carcinoembryonic proteins. The structure of the protein moiety and the biological activities of the AFP in fetal liver and hepatocellular carcinoma (HCC) are almost identical, with only slight differences in the carbohydrate composition. Recently, Aoyagi et al[1] reported a difference in the binding pattern of AFP with lens culinaris agglutinin (LCA) between HCC and benign liver disease (BLD), using the crossed immunoaffinoelectrophoresis (CIAE). An increase of the LCA reactive species of AFP in patients with HCC indicated that fucosylation of the sugar chain was the molecular basis for this AFP variation. However, the CIAE method requires great skill and is, therefore, not appropriate for routine clinical work. The present study describes a clinical application of the two-site sandwich enzyme linked immunosorbent assay (ELISA) assay with anti-human AFP-R-LCA variant monoclonal antibodies (mAb) to detect serum AFP-R-LCA. The results showed that these mAb are clinically useful for the differential diagnosis of HCC and BLD and for increasing the early detection rate of HCC.
A total of 226 cases were included in the study, of which 99 had a histopathological diagnosis of HCC. Thirty patients had tumors < 5 cm in diameter while the rest had tumors > 5 cm in diameter. The serum AFP levels, determined by the rocket immune-electrophoresis (RIE), were < 100 μg/L in 38 (38.4%) patients, < 400 μg/L in 46 (46.4%) patients, and > 400 μg/L in the rest of the patients (15.2%). The serum levels of AFP-R-LCA determined by the CIAE were higher than 25% in 79 patients. AIAT, γ-glutamyl transpeptidase (γ-GT), alkaline phosphatase (ALP), LDH, PHI, and CEA were also detected in HCC patients with tumors < 5 cm in diameter. A total of 67 cases of BLD were included of which 30 had post-hepatitis cirrhosis, 10 acute hepatitis, and 27 chronic active hepatitis. All of these patients received a histopathological diagnosis. A total of 30 pregnant women (3-4 mo in pregnancy) and 30 healthy adult blood donors were taken as controls. AFP levels were determined in all the patients and controls by RIE.
BALB/C mice were immunized with purified AFP-R-LCA (provided by the Hepatobiliary Surgery Institute, Changhai Hospital, the Second Military Medical University). The spleen cells were removed and flushed with FO myeloma cells (Balb/c origin) by 50% PEG methods. After ELISA monitoring and five limit diluting clonozation, five cell lines secreting anti-human AFP-R-LCA mAb were obtained. The titers of the specific mAb, as determined by ELISA, were 2 × 10 in a culture supernatant and 1 × 10 in ascites induced in mice. The hybridoma cell lines were cultured for 5 mo in vitro and passed on for 60 generations. After being frozen in liquid nitrogen and thawed, these cell lines had the ability to secret mAb steadily. Chromosome analysis demonstrated that these cell lines had the characteristics of hybridoma cells. The mAb belonged to IgG class.
The mAb was purified from ascites by diethylaminoethyl cellulose 52 chromatography. The purified mAb and horseradish peroxidase (HRP) were first treated with periodic acid and then precipitated twice with 500 g/L (NH4)2SO4. After an exhaustive dialysis against 0.01 mol/L PBS (pH 7.4), the mixture was then passed through Sephadex G25 column. The HRP-labelled mAb were obtained with a molecular ratio 1.4. The working concentration was 1:2000.
Polystyrene plate was coated overnight with 0.2 mL/well AFP-R-LCA (5 μg/mL). The plate was washed four times with 0.01 mol/L-PBS, followed by addition of 0.1 mL/well of HRP-mAb and 0.2 mL/well of four different ascites (1:10 diluted). The reaction mixtures were incubated for 2 h at 37 °C. Substrate colorization was stopped after 30 min, and O.D. value at 490 nm was determined.
The mAb used for coating did not have cross-reaction with HRP-mAb. Standard curve was drawn for each assay. The accuracy of the ELISA assay had intra-batch coefficients of variability (CV) values (CV = s/x-× 100; s, standard deviation; x-, mean) of 4%, 3.8%, 5.3%, 6.4% and 6.8%, respectively and intra-batch CV values 8.5%, 10.2%, 9.3%, 8.6% and 12.8%, respectively. The obtained accuracy rate was 96.2% ± 5.4%.
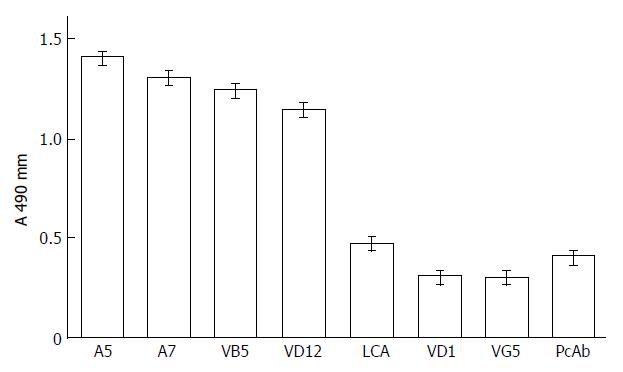
The results of the competitive inhibition assay demonstrated that antigen binding sites of anti-AFP mAb A5 and A7 and anti-AFP-R-LCA mAb VB5 and VD12 were different from those of VD1 and VG5 (Figure 1). VD1 and VG5 may bind the same antigenic sites, or the antigenic sites they bound were very close to each other. Polyclonal horse anti-human AFP antibodies and LCA also inhibited the binding of HRP-mAb VG5 to AFP-R-LCA. The presence of two different mAb with no cross-reactivity in the prepared anti-AFP-R-LCA formed the basis of the two-site sandwich ELISA assay.
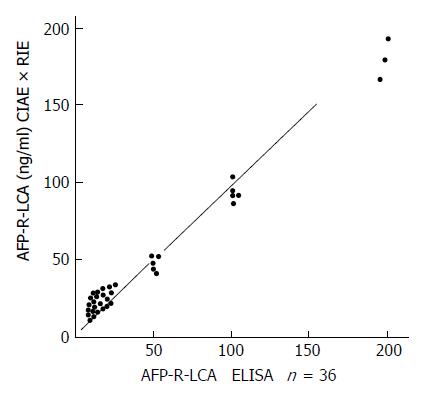
Serum AFP-R-LCA was determined by the two-site sandwich ELISA assay. Serum AFP and the ratio of AFP/AFP-R-LCA were determined by RIE and CIAE. The correlation of the two-site sandwich ELISA with RIE × CIAE was analyzed in the curve of ELISA against RIE × CIAE. There was a significant positive correlation between the results of ELISA and those of RIE × CIAE as demonstrated in Figure 2 (γ = 0.8401, P < 0.01).
AFP-R-LCA value for pregnant women and normal controls were tested, and the concentration of AFP-R-LCA in serum was 1.27 ± 0.6 μg/L and 0 μg/L, respectively. Concerning the previously published paper, we set the threshold value of serum AFP-R-LCA to 10 μg/L. No false negatives were observed, and the false positive was only 1.45%.
The results of the eight parameters obtained from 30 patients with small HCC are shown in Table 1. The range of serum AFP concentration was 0 μg/L–2000 μg/L. When > 400 μg/L of AFP was taken as a positive threshold, 10 patients were positive (33.3%) for AFP. The concentration range for AFP-R-LCA was 0 μg/L-300 μg/L in these patients. When AFP-R-LCA threshold was set at > 10 μg/L, 26 patients were positive. None of the other six parameters had a positive rate larger than 30%. In four patients, no AFP-R-LCA could be detected, so these patients were regarded as AFP-negative primary liver cancer.
| Item | Number | Concentration | Positive rate %1 |
| AFP | 30 | 0-2000 μg/L | 33.3 |
| AFP-R-LCA | 30 | 0-300 μg/L | 86.7 |
| AIAT | 28 | 250-560 μg/L | 14.3 |
| γ-GT | 30 | 20-420 U | 25.0 |
| ALP | 30 | 6-28 U | 13.3 |
| ADH | 25 | 120-860 U | 28.0 |
| PHI | 25 | 23-216 U | 20.0 |
| CEA | 14 | 6.8-62.7 U | 21.4 |
The results showed that serum AFP positive rate was increased from 62.6% (RIE) to 86.8% (two-site sandwich ELISA assay) in HCC patients while the positive rates were decreased from 19.4% to 1.45% in BLD (P < 0.05) and from 26.7% to 0% in pregnant women. The two-site sandwich ELISA assay had a specificity of 99.0% in the diagnosis of HCC (Table 2).
| Patients | n | Positive rate of AFP (%) | Positive rate of AFP-R-LCA (%) |
| HCC | 99 | 62.6 | 86.8 |
| BLD | 67 | 19.4 | 1.45 |
| Pregnant women | 30 | 26.7 | 0 |
| Normal controls | 30 | 0 | 0 |
In 46 HCC patients with AFP 400 μg/L and 38 HCC patients with AFP 100 μg/L, the AFP-R-LCA concentrations were 36.8 ± 10.2 μg/L and 19.1 ± 10.4 μg/L, respectively. In 54 BLD patients with AFP 400 μg/L and 51 BLD patients with AFP 100 μg/L, the AFP-R-LCA concentrations were 4.40 ± 2.3 μg/L and 1.91 ± 0.9 μg/L, respectively (P < 0.01). In 30 pregnant women, the AFP-R-LCA concentration was 1.27 ± 0.6 μg/L (P < 0.01). The above results indicate that although HCC patients may have AFP levels lower than the positive threshold, the AFP-R-LCA concentrations were higher than the positive threshold and also much higher than that in BLD patients.
As of recently, more attention has been paid to carbohydrates as “functional codes”. Since lectins can specifically bind to carbohydrates, they have been widely used in the study of the heterogeneity of AFP carbohydrate chains. Consequently, much progress has been made in the field. In the current study, Du et al[2] found that the reactivity of serum AFP from 20 patients with HCC with immobilized lentil-lectin was significantly greater (39% ± 18%) than that of the same protein from patients with chronic liver disease (11.2% ± 3.3%), fulminant hepatic failure (10% ± 8.4%), and normal pregnant women (4.1% ± 2.7%). However, in these experiments the lack of correlation between the ConA and lentil-lectin reactivities of AFP in HCC suggests that the implied structure changes involving D-mannose/D-glucose and L-fucose respectively, resulting from quite distinct cellular lesions.
Aoyagi et al[3] has purified LCA reactive and non-reactive heterogeneous AFP variants from HCC ascites and analyzed their carbohydrate chain structure. Their results showed that LCA-reactive AFP has N-acetyl 1-glucose residue bound to Asn, and this residue has a bound fucose residue. The group tested the sera from the patients with HCC and found that the ratio of LCA-R-AFP heterogeneous variant significantly increased. This may be a result of decreased fucosidase activity. Since the fucosidase activity decreases, the fucose residue bound to G1cNAc can not be removed during the process of AFP carbohydrate chain modification, and so forms the characteristic structure that can be recognized by LCA.
The degree of AFP fucosylation has been revealed as a good marker for discrimination of HCC from nonmalignant liver diseases[4]. The fucosylated and non-fucosylated molecular species of AFP have been identified by CIAE in the presence of LCA, by taking advantage of the affinity of the fucosylated species with this lectin. Similar methods have been used for diagnosis of neural tube defects and liver disease with lectins such as concanavalin A and LCA[5]. However, the methods using CIAE, immunoblotting techniques, and affinity chromatography are complicated and require a high level of technical skill.
Several investigators have described the application of mAb assay systems as quantitative measurement of serum markers in patients with certain disease[6]. In recent years, many studies have demonstrated that mAb can immunologically distinguish among AFP from different organs. At least 10 mAb against AFP have been reported, and their introduction increased the detection sensitivity and specificity. However, these antibodies still could not distinguish BLD from HCC for these two AFPs belong to the same fetal liver type. Thus, it was necessary to develop mAb that can specifically recognize the relatively specific reverse differential protein AFP-R-LCA induced by transformed cells in HCC for the early diagnosis of HCC and the differential diagnosis of BLD and HCC. Suzuki et al[7] described a new enzyme immunoassay (EIA) that distinguished between purified fucosylated and non-fucosylated AFP using monoclonal anti-AFP antibody in the presence of LCA at AFP concentration from 200 μg/L to 1000 μg/L. They suggested that their new EIA with mAb18H4 would be useful for detection of an early stage of HCC. However, a great quantity of LCA is consumed in EIA, and economic load on patients would increase consequently. On the other hand, our anti-AFP-R-LCA mAbs have high specificity and affinity, especially mAbs VG5 and VD1, which recognize new antigenic sites (different from the already known AFP a and b antigenic sites). The two-site sandwich ELISA assay set up using these two mAbs can quantitatively analyze the AFP-R-LCA concentration and increase the detection rate of HCC. This ELISA assay is superior to the CIAE, which is currently widely used in clinical studies. The results of our study show that 10 μg/L of AFP-R-LCA is a reasonable threshold. Based on this threshold, the detection rate was 86.8% for HCC, 86.7% for small HCC, and 100% for AFP positive HCC. No false negatives were encountered. Only one BLD patient had serum AFP-R-LCA higher than 10 μg/L (false positive rate, 1.45%). No one in pregnant women and control groups had AFP-R-LCA higher than 10 μg/L. In HCC patients with low AFP levels, 46 cases (46.6%) had AFP levels less than the positive threshold of 400 μg/L. However, the mean AFP-R-LCA concentration was 36.8 ± 10.2 μg/L in these patients, much higher than the positive threshold. When AFP levels were determined by RIE, the positive rate was 62.9%, and the false positive rate was 19.4% for HCC. Since six BLD patients and 53 HCC patients had AFP of 400 μg/L, the coincidence of diagnosis was 89.9% (53/59). The detection rate for HCC was 79.7% (79/99) by CIAE. CIAE and RIE had lower sensitivity and in accuracy than ELISA assay.
We suggest from this study that the quantitative analysis of serum AFP-R-LCA is useful not only in increasing the early diagnosis rate and accuracy of HCC with low AFP but also for the early diagnosis of small HCC. At present, it is difficult to distinguish HCC from AFP-positive BLD in a clinical setting. If the two-site sandwich ELISA assay is used to analyze quantitatively AFP-R-LCA concentration, the accuracy of differential diagnosis of HCC and BLD will increase and enable patients to gain the opportunity of an early treatment. The mAbs are easy to prepare and have higher specificity than polyclonal antibodies. Combining mAb with ELISA provides a simple, fast, and sensitive method which can be used widely in clinical practice. It is possible that this mAb two-site sandwich ELISA assay will replace the routine methods for AFP analysis.
| 1. | Aoyagi Y, Isemura M, Suzuki Y, Sekine C, Soga K, Ozaki T, Ichida F. Change in fucosylation of alpha-fetoprotein on malignant transformation of liver cells. Lancet. 1986;1:210. [PubMed] |
| 2. | Du MQ, Hutchinson WL, Johnson PJ, Williams R. Differential alpha-fetoprotein lectin binding in hepatocellular carcinoma. Diagnostic utility at low serum levels. Cancer. 1991;67:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Aoyagi Y, Suzuki Y, Isemuse M, Soga K, Ozaki T and Ichida T. Molecular differentiation of alphafetoprotein from hepatocellular carcinoma and from non neoplastic liver disease (in Japamese). Kantansui. 1985;11:73-79. |
| 4. | Aoyagi Y, Suzuki Y, Isemura M, Nomoto M, Sekine C, Igarashi K, Ichida F. The fucosylation index of alpha-fetoprotein and its usefulness in the early diagnosis of hepatocellular carcinoma. Cancer. 1988;61:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Taketa K, Ichikawa E and Rage H. Antibodya2affinity blotting, a sensitive technique for the detection of alphafetoprotein separated by lectin affinitya2electrophoresis in agarose gels. Electrophoresis. 1985;6:492-497. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Kuroki M, Arakawa F, Higuchi H, Matsunaga A, Okamoto N, Takakura K, Matsuoka Y. Epitope mapping of the carcinoembryonic antigen by monoclonal antibodies and establishment of a new improved radioimmunoassay system. Jpn J Cancer Res. 1987;78:386-396. [PubMed] |
| 7. | Suzuki Y, Aoyagi Y, Muramatsu M, Igarashi K, Saito A, Oguro M, Isemura M, Asakura H. A lectin-based monoclonal enzyme immunoassay to distinguish fucosylated and non-fucosylated alpha-fetoprotein molecular variants. Ann Clin Biochem. 1990;27:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Zhang FF