©The Author(s) 2026.
World J Gastroenterol. Feb 7, 2026; 32(5): 115301
Published online Feb 7, 2026. doi: 10.3748/wjg.v32.i5.115301
Published online Feb 7, 2026. doi: 10.3748/wjg.v32.i5.115301
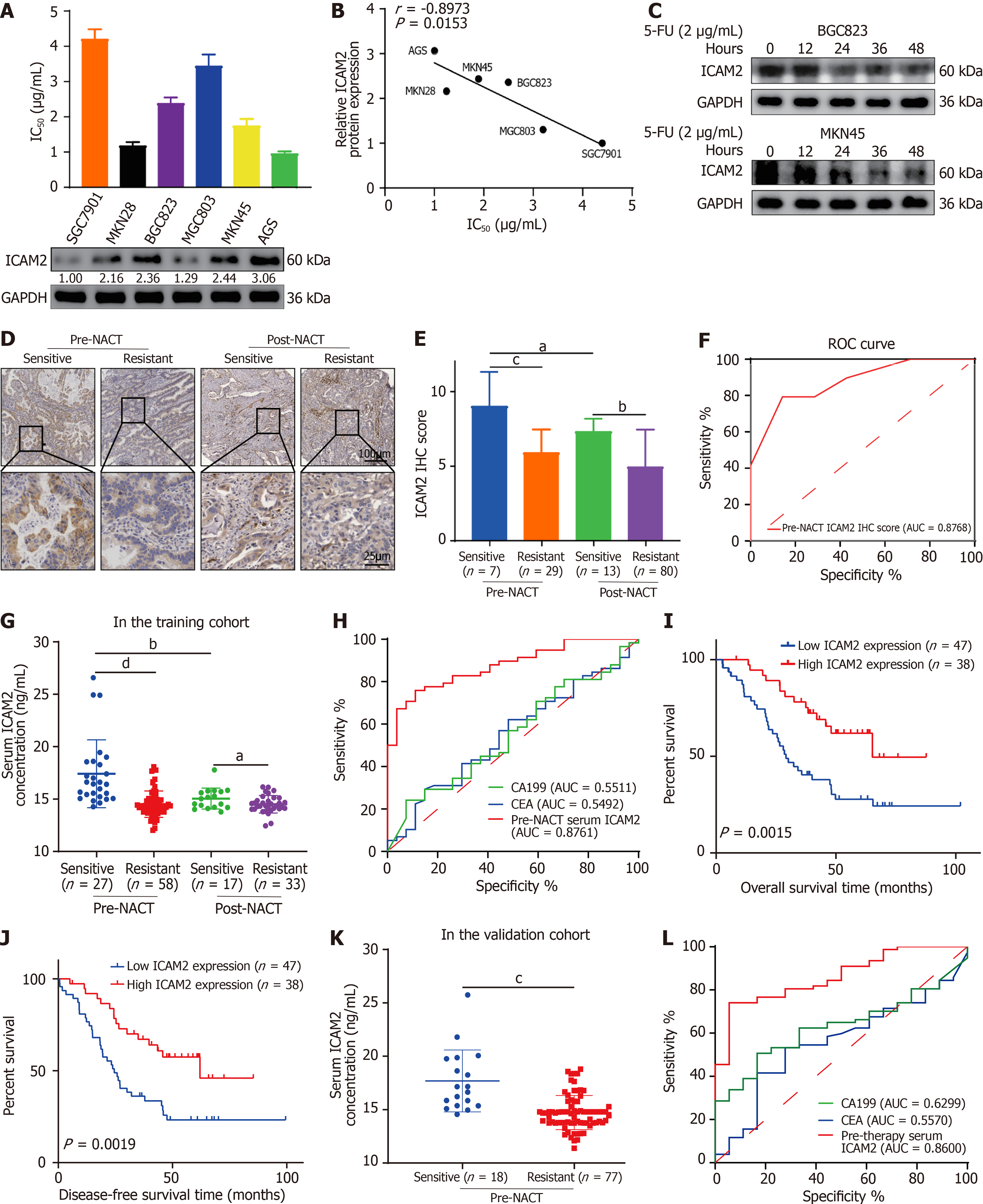
Figure 1 Intercellular adhesion molecule 2 expression correlates with 5-fluorouracil resistance and predicts neoadjuvant chemotherapy response in advanced gastric cancer patients.
A and B: Correlation between 5-fluorouracil (5-FU) half-maximal inhibitory concentration values and intercellular adhesion molecule 2 (ICAM2) protein expression in the gastric cancer (GC) cell lines; C: Time-dependent changes in ICAM2 protein levels following 5-FU treatment; D and E: Immunohistochemistry analysis of ICAM2 expression in chemo-sensitive and chemo-resistant advanced GC (AGC) patients before and after neoadjuvant chemotherapy (NACT); F: ROC curve based on pre-NACT tumor tissue ICAM2 levels to differentiate between chemo-sensitive and chemo-resistant patients; G: Enzyme-linked immunosorbent assay (ELISA) analysis of serum ICAM2 levels in the training cohort, comparing chemo-sensitive and chemo-resistant patients before and after NACT; H: Receiver-operating characteristic (ROC) curve based on pre-NACT serum ICAM2 concentrations to predict NACT response; I and J: Kaplan-Meier survival curves showing overall survival and disease-free survival for AGC patients with low or high pre-NACT serum ICAM2 levels; K: ELISA analysis of serum ICAM2 levels in the validation cohort, comparing chemo-sensitive and chemo-resistant patients prior to NACT; L: ROC curve analysis using pre-NACT serum ICAM2 levels to distinguish between chemo-sensitive and chemo-resistant patients. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001. 5-FU: 5-fluorouracil; IC50: Half-maximal inhibitory concentration; ICAM2: Intercellular adhesion molecule 2; NACT: Neoadjuvant chemotherapy; ROC: Receiver-operating characteristic; AUC: Area under the curve; ELISA: Enzyme-linked immunosorbent assay; CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen.
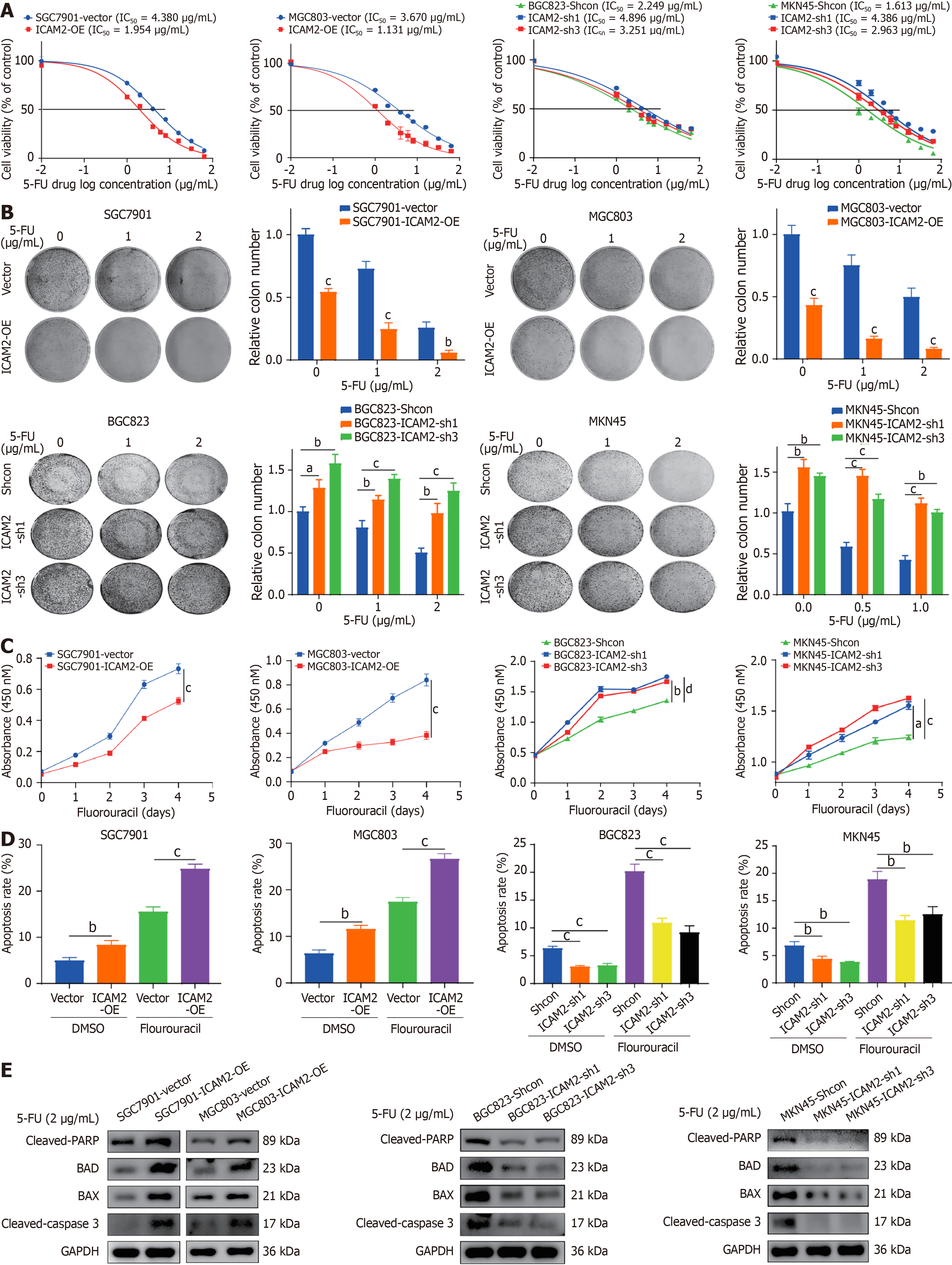
Figure 2 Intercellular adhesion molecule 2 knockdown enhances 5-fluorouracil resistance in gastric cancer cells by inhibiting caspase-dependent apoptosis.
A: Half-maximal inhibitory concentration values for 5-fluorouracil (5-FU) in gastric cancer (GC) cells with intercellular adhesion molecule 2 (ICAM2) overexpression or knockdown; B: Colony formation assay in GC cells treated with varying 5-FU concentrations after ICAM2 overexpression or silencing; C: Growth curves of GC cells with ICAM2 overexpression or knockdown cultured with 5-FU; D: Flow cytometric analysis of apoptosis in 5-FU-treated GC cells with ICAM2 overexpression or knockdown; E: Western blotting analysis of apoptosis-related proteins in 5-FU-treated GC cells with ICAM2 overexpression or knockdown. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001. 5-FU: 5-fluorouracil; IC50: Half-maximal inhibitory concentration; ICAM2: Intercellular adhesion molecule 2.
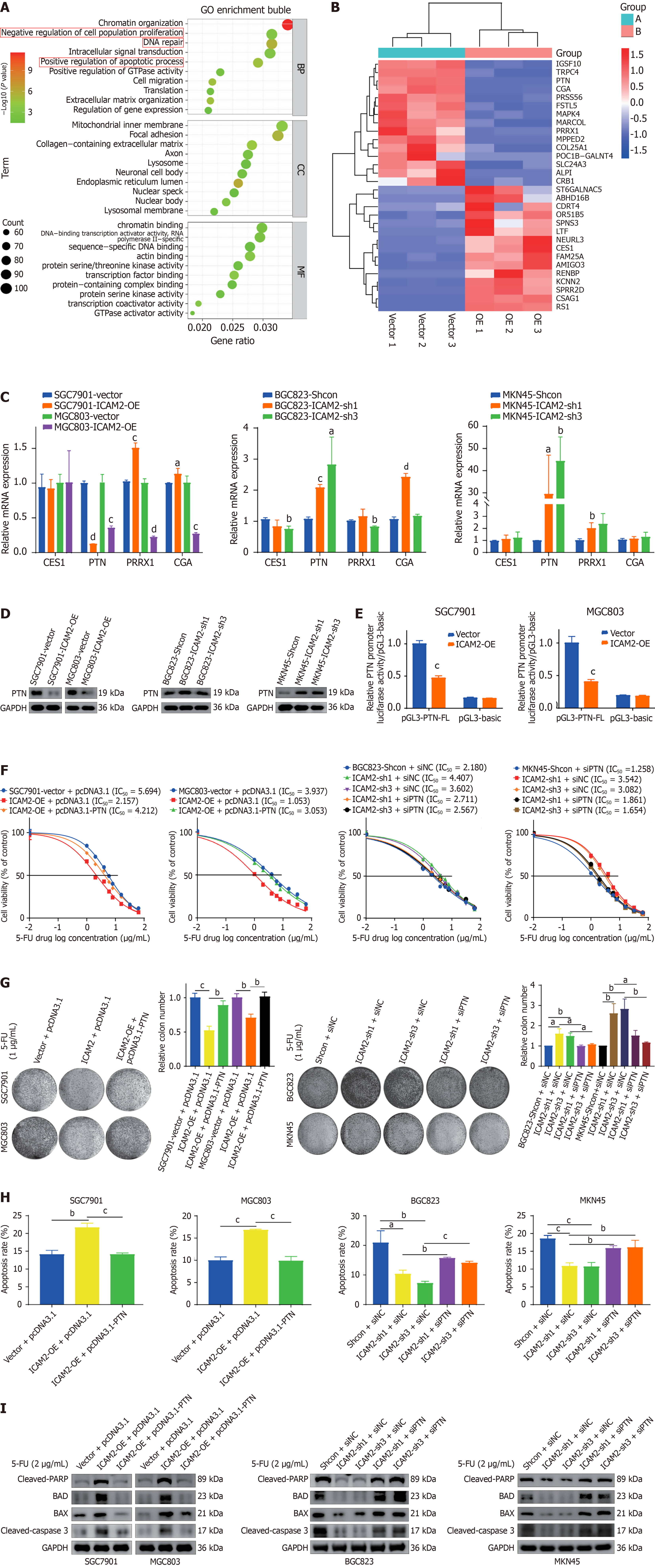
Figure 3 Pleiotrophin mediates intercellular adhesion molecule 2-driven chemoresistance to 5-fluorouracil in gastric cancer.
A: Gene Ontology enrichment analysis of differentially expressed genes (DEGs); B: Heatmap showing expression patterns of DEGs between control and intercellular adhesion molecule 2 (ICAM2)-overexpressing cells (red = upregulated, blue = downregulated, based on z-score of normalized expression); C: Real-time PCR validation of DEG expression; D: Changes in pleiotrophin (PTN) protein expression following ICAM2 overexpression or knockdown; E: PTN promoter activity assessed in the indicated cell models using a dual-luciferase reporter assay; F-I: Cytotoxicity, colony formation, flow cytometry, and apoptosis-related protein assays evaluating the reversal of ICAM2-mediated 5-fluorouracil resistance by PTN overexpression or silencing. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001. PTN: Pleiotrophin; 5-FU: 5-fluorouracil; IC50: Half-maximal inhibitory concentration; ICAM2: Intercellular adhesion molecule 2; GO: Gene Ontology.
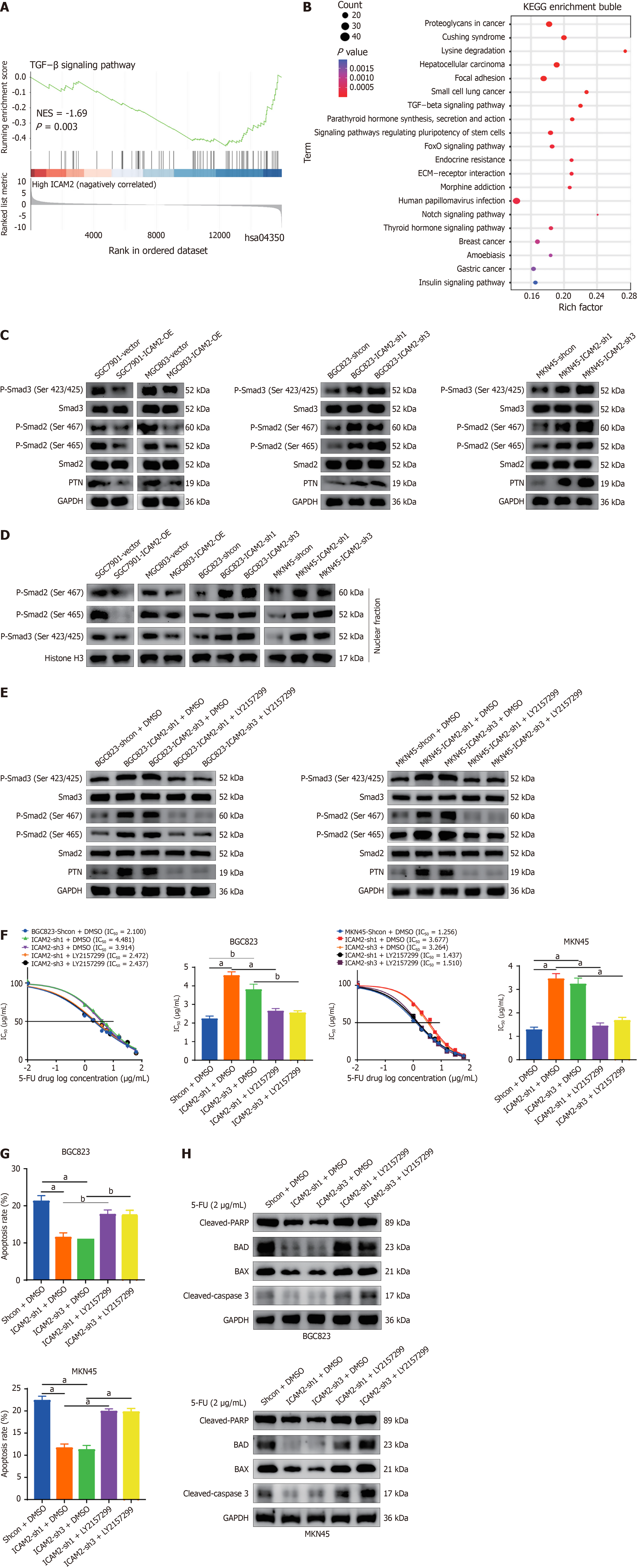
Figure 4 Intercellular adhesion molecule 2 knockdown enhances pleiotrophin expression and 5-fluorouracil resistance by activating the TGF-β/Smad signaling in gastric cancer.
A: The relationship between the TGF-β signaling pathway and intercellular adhesion molecule 2 (ICAM2) in gene set enrichment analysis based on RNA-seq data; B: Kyoto Encyclopedia of Genes and Genomes pathway analysis showing enrichment of the TGF-β pathway; C: Western blot analysis of TGF-β/Smad2/3 pathway proteins and pleiotrophin (PTN) expression in the indicated cells; D: Immunoblotting of nuclear P-Smad2/3 levels in the indicated cells; E: Treatment with LY2157299 reversed the effects of ICAM2 knockdown on TGF-β/Smad2/3 pathway proteins and PTN expression; F-H: Cytotoxicity, flow cytometry, and apoptosis-related protein analysis of ICAM2-knockdown gastric cancer cells treated with DMSO or LY2157299. aP < 0.001; bP < 0.01. PTN: Pleiotrophin; 5-FU: 5-fluorouracil; KEGG: Kyoto Encyclopedia of Genes and Genomes; ICAM2: Intercellular adhesion molecule 2; IC50: Half-maximal inhibitory concentration.
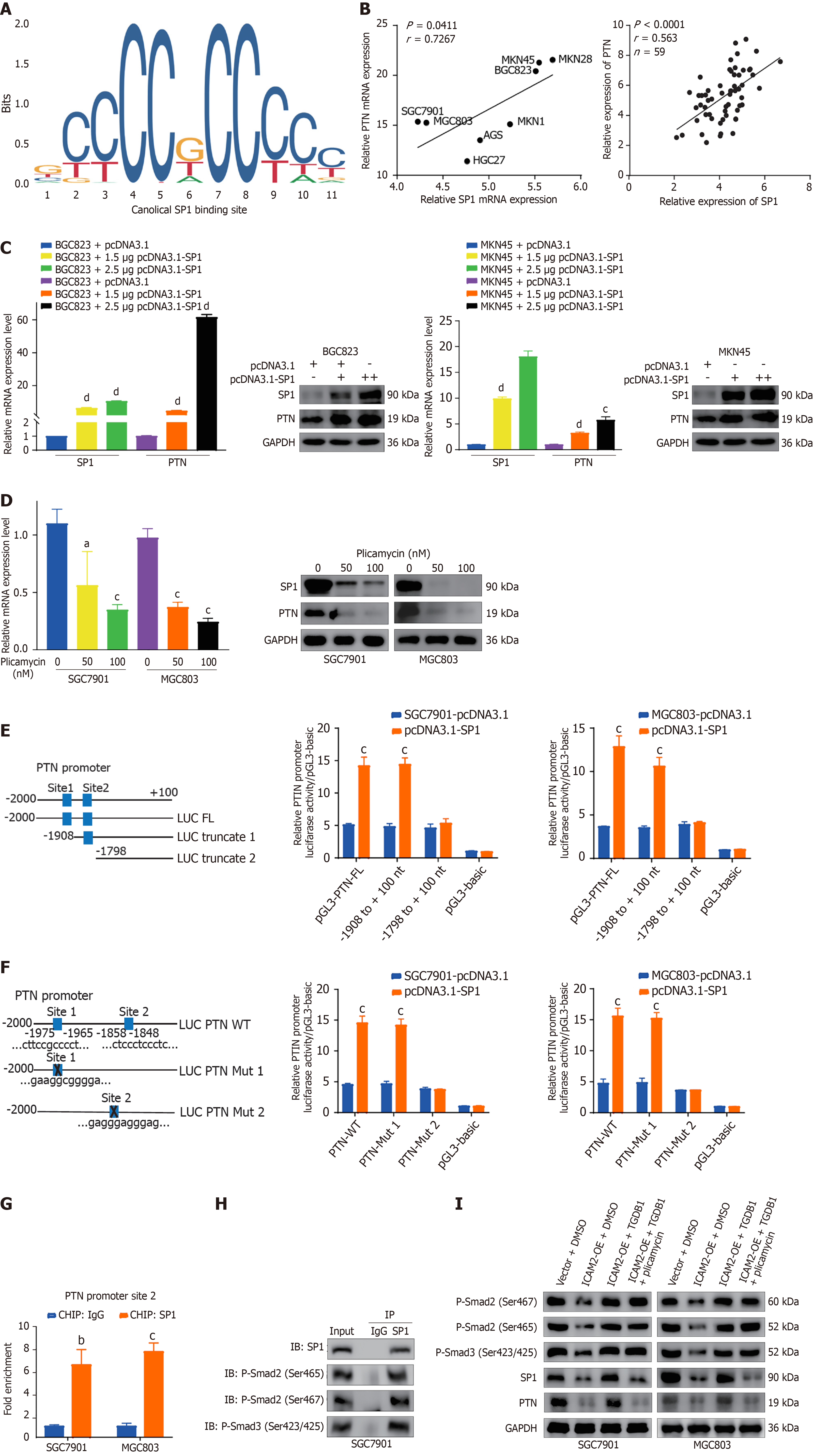
Figure 5 SP1 directly regulates pleiotrophin transcription and mediates TGF-β/Smad signaling in gastric cancer chemoresistance.
A: Prediction of SP1 binding motifs and sites within the pleiotrophin (PTN) promoter region using the JASPAR database; B: Correlation analysis between SP1 and PTN mRNA levels in gastric cancer (GC) cell lines and patient tissue samples; C and D: Immunoblotting and real-time PCR analysis to assess the effect of SP1 overexpression or silencing on PTN expression; E: SP1 binding sites (sites 1 and 2) in the PTN promoter. Luciferase reporter assays demonstrated that SP1 specifically enhanced activity of pGL3-PTN-FL and pGL3-PTN-Truncated 1 promoters; F: Schematic representation of mutations in PTN Mut 1 and Mut 2 plasmids. SP1 failed to activate PTN Mut 2 due to the absence of site 2; G: Chromatin immunoprecipitation (ChIP) assays confirmed direct binding of SP1 to site 2 within the PTN promoter; H: Co-IP assays performed to detect interactions between SP1, P-Smad2, and P-Smad3 in GC cells; I: Immunoblotting analysis of intercellular adhesion molecule 2-overexpressing cells treated with TGF-β1 with or without plicamycin, showing changes in P-Smad2/3, SP1, and PTN levels. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001. PTN: Pleiotrophin.
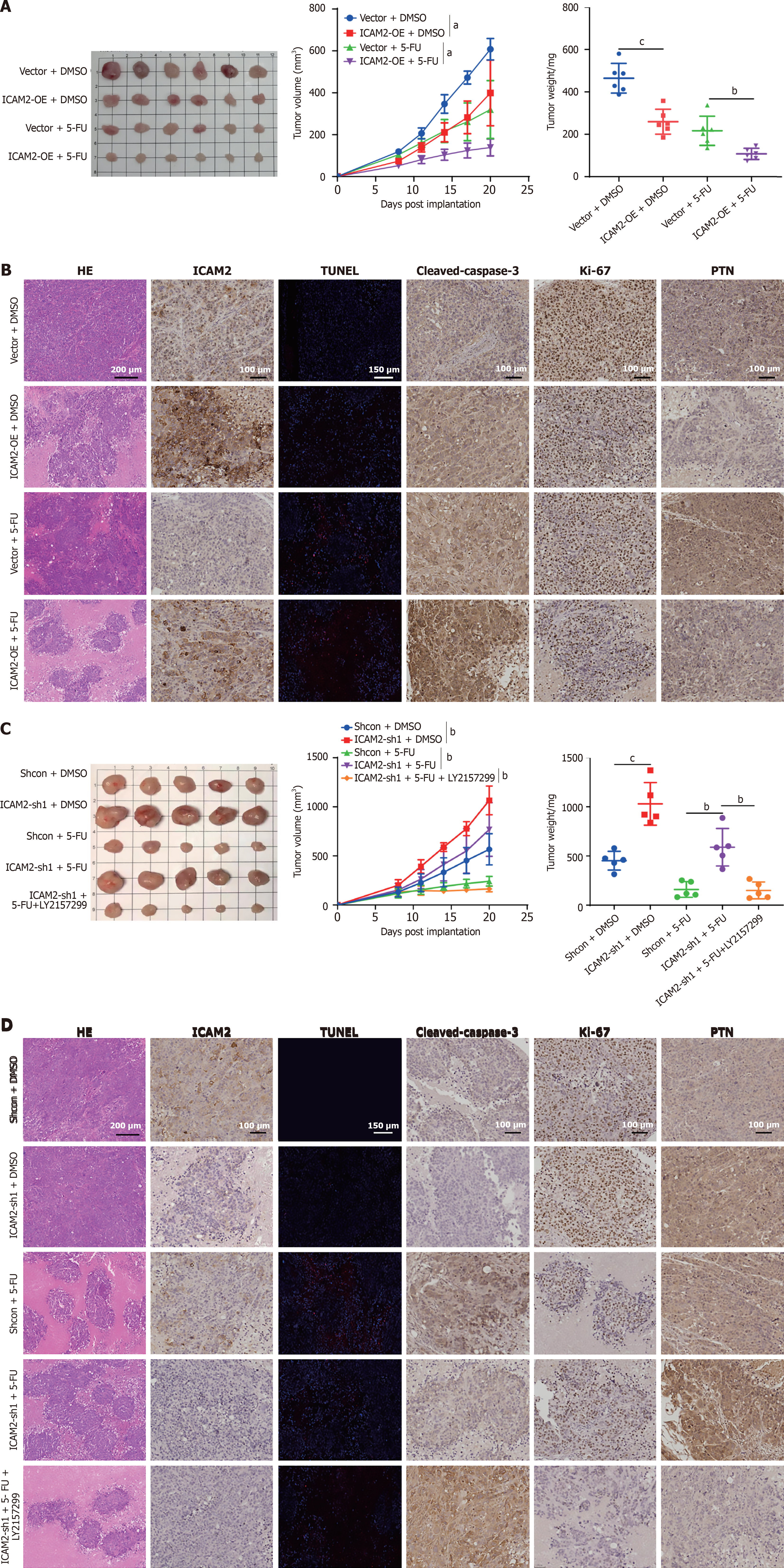
Figure 6 Targeting TGF-β/Smad signaling overcomes 5-fluorouracil resistance in gastric cancer with low intercellular adhesion molecule 2 expression.
A: Representative tumor images and tumor volume changes in mice bearing MGC803 xenografts treated with vector + DMSO, intercellular adhesion molecule 2 (ICAM2)-OE + DMSO, vector + 5-fluorouracil (5-FU), and ICAM2-OE + 5-FU. Tumor weights were measured on day 22 (n = 6/group); B: Immunohistochemistry (IHC) staining for ICAM2, Ki-67, pleiotrophin (PTN), and cleaved caspase-3, along with TUNEL staining in tumors from the indicated groups; C: Representative tumor images and tumor volume changes in mice bearing MKN45 xenografts treated with Shcon + DMSO, ICAM2-sh1 + DMSO, Shcon + 5-FU, ICAM2-sh1 + 5-FU, and ICAM2-sh1 + 5-FU + LY2157299. Tumor weights were measured on day 22 (n = 5/group); D: IHC staining for ICAM2, Ki-67, PTN, and cleaved caspase-3, along with TUNEL staining in tumors from the indicated groups. aP < 0.05; bP < 0.01; cP < 0.001. 5-FU: 5-fluorouracil; ICAM2: Intercellular adhesion molecule 2; PTN: Pleiotrophin.
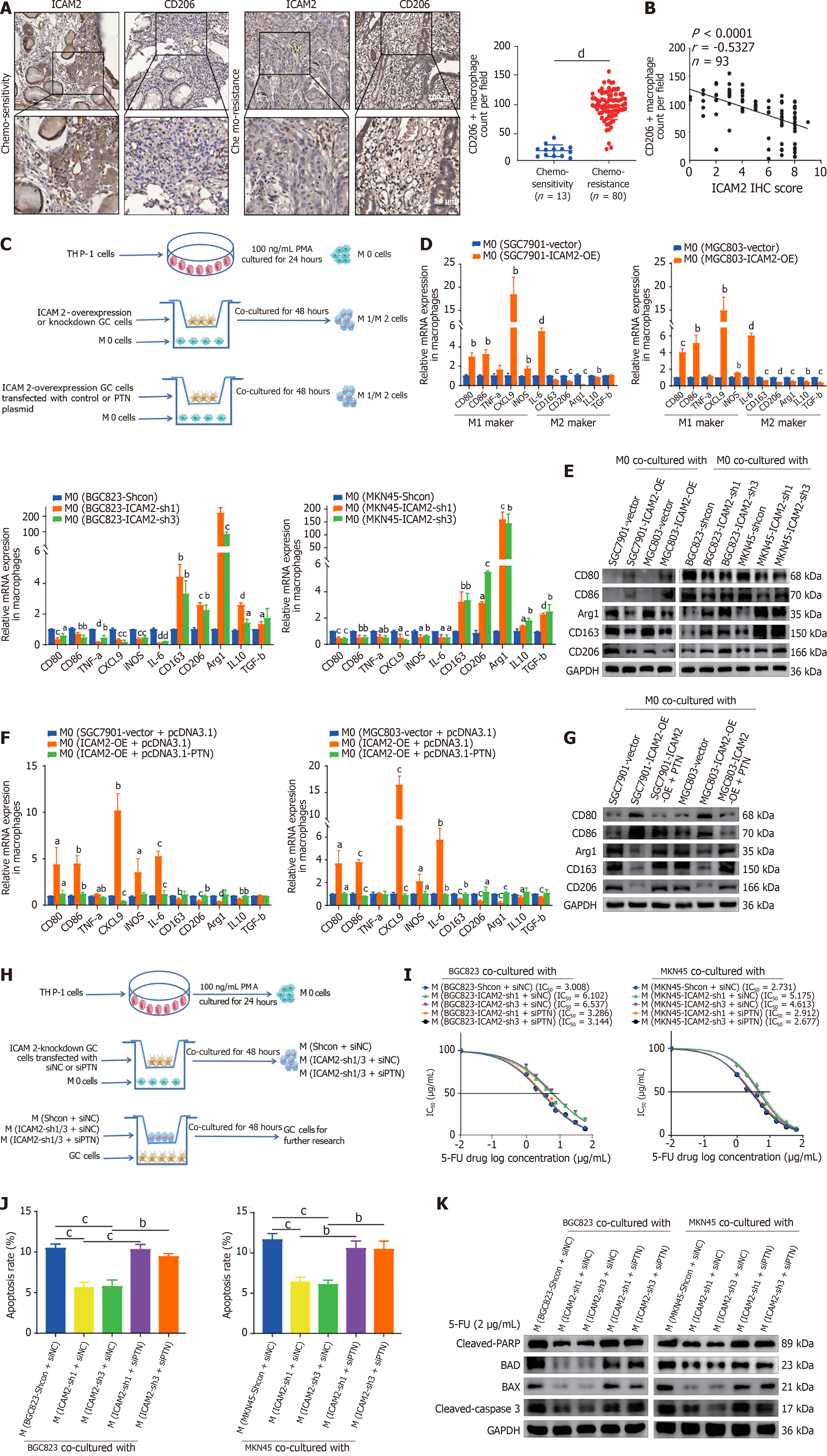
Figure 7 Intercellular adhesion molecule 2 downregulation promotes M2 macrophage polarization potentially involving pleiotrophin, driving 5-fluorouracil resistance in gastric cancer.
A: Representative immunohistochemistry images showing intercellular adhesion molecule 2 (ICAM2) and CD206 expression in chemo-resistant and chemo-sensitive advanced gastric cancer (AGC) tissues. M2 macrophage infiltration was significantly higher in chemo-resistant tissues; B: Correlation analyses between M2 macrophages infiltration and ICAM2 expression in AGC patient specimens; C: Overview of the differentiation protocol used to induce THP-1 cells into M0 macrophages; D: Real-time PCR (RT-PCR) analysis of M1-related (CD80, CD86, TNF-α, CXCL9, iNOS, IL-6) and M2-related (CD163, CD206, IL-10, Arg-1, TGF-β) gene expression in macrophages; E: Western blotting analysis of M1 (CD80, CD86) and M2 (Arg1, CD163, CD206) protein levels in macrophages; F and G: RT-PCR and Western blotting analyses evaluating the potential role of pleiotrophin (PTN) in influencing ICAM2-mediated macrophage polarization; H: Schematic of the transwell system used to investigate the impact of macrophage polarization on GC cell chemoresistance; I-K: Cytotoxicity assays, flow cytometry, and analysis of apoptosis-related proteins assessing the potential involvement of PTN in modulating the effects of ICAM2-induced M2 macrophage polarization on 5-fluorouracil resistance in GC cells. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001. ICAM2: Intercellular adhesion molecule 2; PTN: Pleiotrophin; 5-FU: 5-fluorouracil; IC50: Half-maximal inhibitory concentration.
- Citation: Tang XC, Chen ZJ, Chen CY, Qiu J, Huang JT, Tan RC, Li WY, Chen H, Yang ZL. ICAM2 loss drives 5-fluorouracil resistance via TGF-β/Smad/SP1/PTN-dependent apoptosis evasion and macrophage remodeling in gastric cancer. World J Gastroenterol 2026; 32(5): 115301
- URL: https://www.wjgnet.com/1007-9327/full/v32/i5/115301.htm
- DOI: https://dx.doi.org/10.3748/wjg.v32.i5.115301