©The Author(s) 2025.
World J Gastroenterol. Oct 21, 2025; 31(39): 110971
Published online Oct 21, 2025. doi: 10.3748/wjg.v31.i39.110971
Published online Oct 21, 2025. doi: 10.3748/wjg.v31.i39.110971
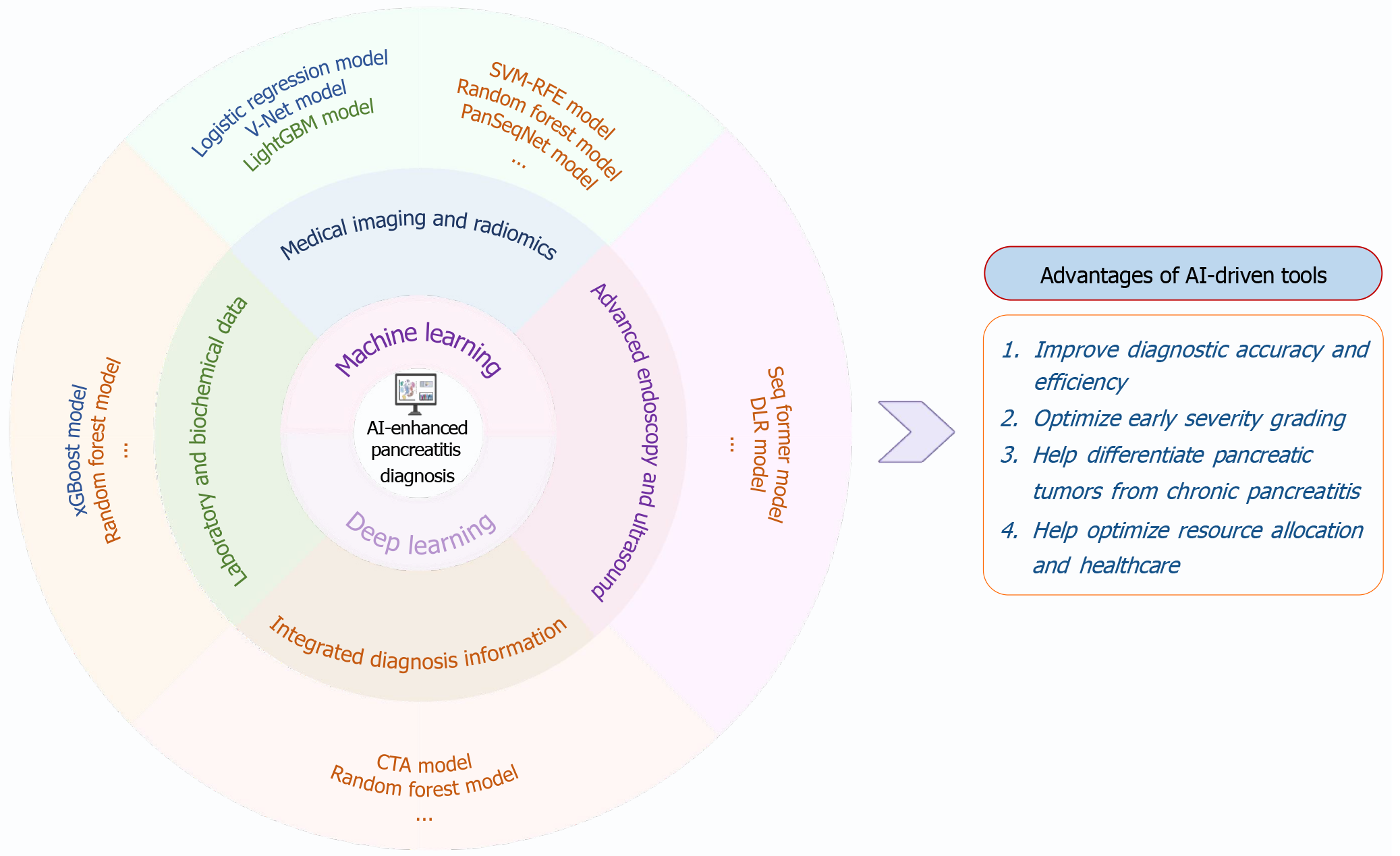
Figure 1 Schematic overview of high-accuracy artificial intelligence models for the precision diagnosis of pancreatitis and their clinical advantages.
Artificial intelligence-enhanced diagnostic tools, encompassing machine learning and deep learning algorithms, integrate multimodal data, ranging from laboratory and biochemical markers to radiologic imaging, endoscopic ultrasound, and computed tomography angiography. Representative models include random forest, XGBoost, SVM-RFE, LightGBM, V-Net, SegFormer, deep learning radiomics, PanSegNet, and others. These approaches have demonstrated utility in improving diagnostic accuracy and efficiency, enabling early severity stratification, differentiating pancreatic neoplasms from chronic pancreatitis, and informing more effective allocation of healthcare resources. The implementation status of each model is indicated by color coding: Experimental (blue), validation phase (orange), or clinically deployed (green). AI: Artificial intelligence; LightGBM: Light gradient boosting machine; CTA: Computed tomography angiography; SVM-RFE: Support vector machine-recursive feature elimination; DLR: Deep learning radiomics.
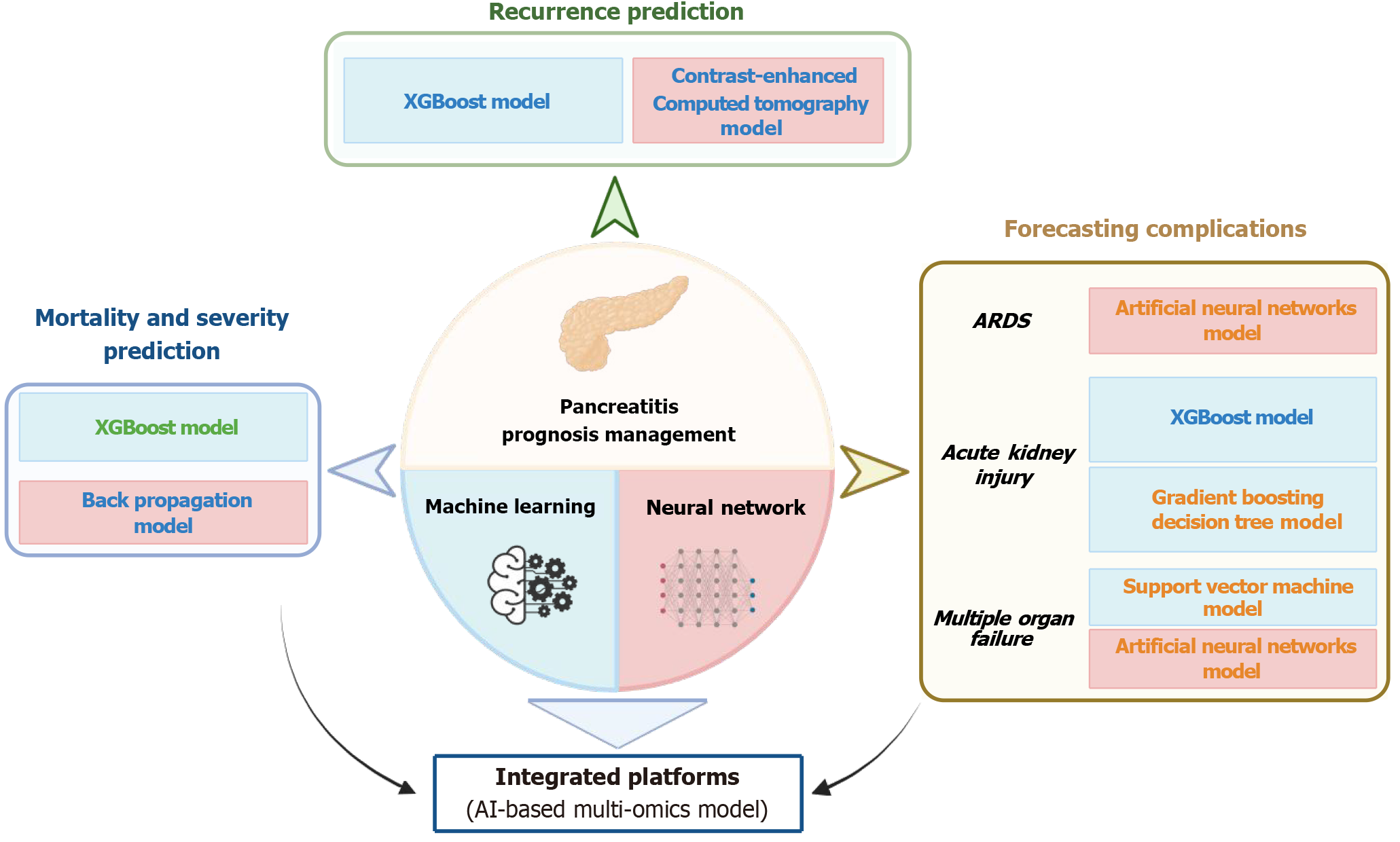
Figure 2 Representative artificial intelligence models for prognosis management in pancreatitis.
A range of machine learning and neural network-based algorithms have been applied to predict recurrence, assess disease severity and mortality risk, and forecast complications such as acute respiratory distress syndrome, acute kidney injury, and multiple organ failure. Notable models include XGBoost, gradient boosting decision tree, back propagation, support vector machine, and feed-forward neural networks. These models, particularly when integrated into multi-omics platforms, enable individualized prognostication and support precision management strategies in clinical practice. The implementation status of each model is indicated by color coding: Experimental (blue), validation phase (orange), or clinically deployed (green). ARDS: Acute respiratory distress syndrome; AI: Artificial intelligence.
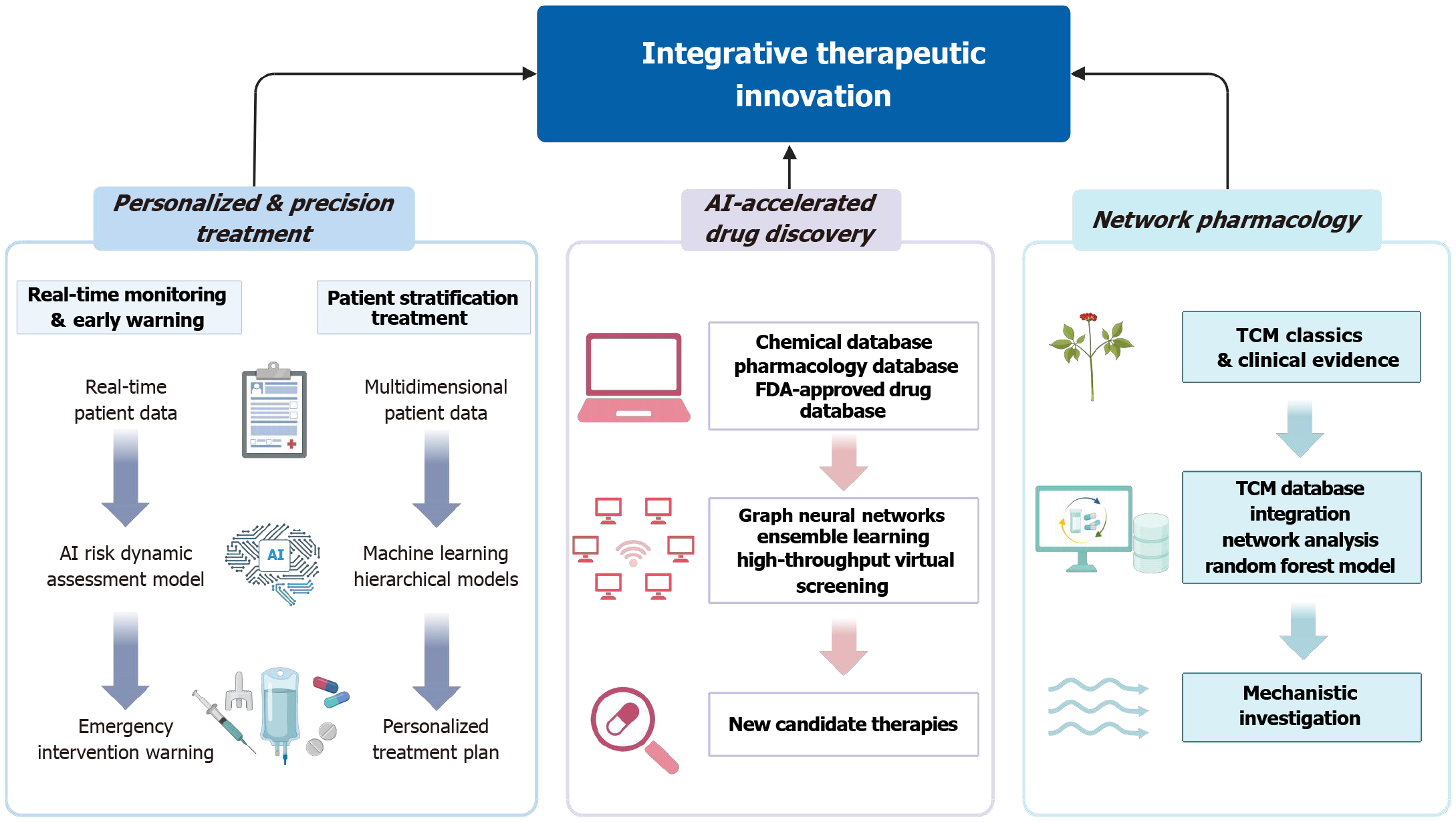
Figure 3 Artificial intelligence-driven strategies for therapeutic innovation in pancreatitis.
Artificial intelligence (AI) facilitates precision treatment through clinical decision support systems that integrate multimodal patient data to optimize resuscitation, nutritional support, pain management, and intensive care unit triage. Machine learning algorithms dynamically update risk profiles and stratify patients to guide individualized interventions. AI is also accelerating drug discovery and repositioning by enabling high-throughput screening of chemical libraries, predicting compound-target interactions, and identifying repurposable agents with anti-inflammatory or antifibrotic potential. Additionally, network pharmacology approaches powered by AI are uncovering bioactive compounds and mechanistic targets within traditional Chinese medicine formulations, bridging empirical practices with molecular pharmacology. These integrative frameworks represent a paradigm shift in pancreatitis care, offering data-driven insights into both modern and traditional therapeutic avenues. AI: Artificial intelligence; FDA: Food and Drug Administration; TCM: Traditional Chinese medicine.
- Citation: Zhang XY, Hu MD, Maimaitijiang D, Wang T, Wang L. Artificial intelligence in pancreatitis: A narrative review on advancing precision diagnosis, prognosis, and therapeutic strategies. World J Gastroenterol 2025; 31(39): 110971
- URL: https://www.wjgnet.com/1007-9327/full/v31/i39/110971.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i39.110971