©The Author(s) 2025.
World J Gastroenterol. Oct 7, 2025; 31(37): 111914
Published online Oct 7, 2025. doi: 10.3748/wjg.v31.i37.111914
Published online Oct 7, 2025. doi: 10.3748/wjg.v31.i37.111914
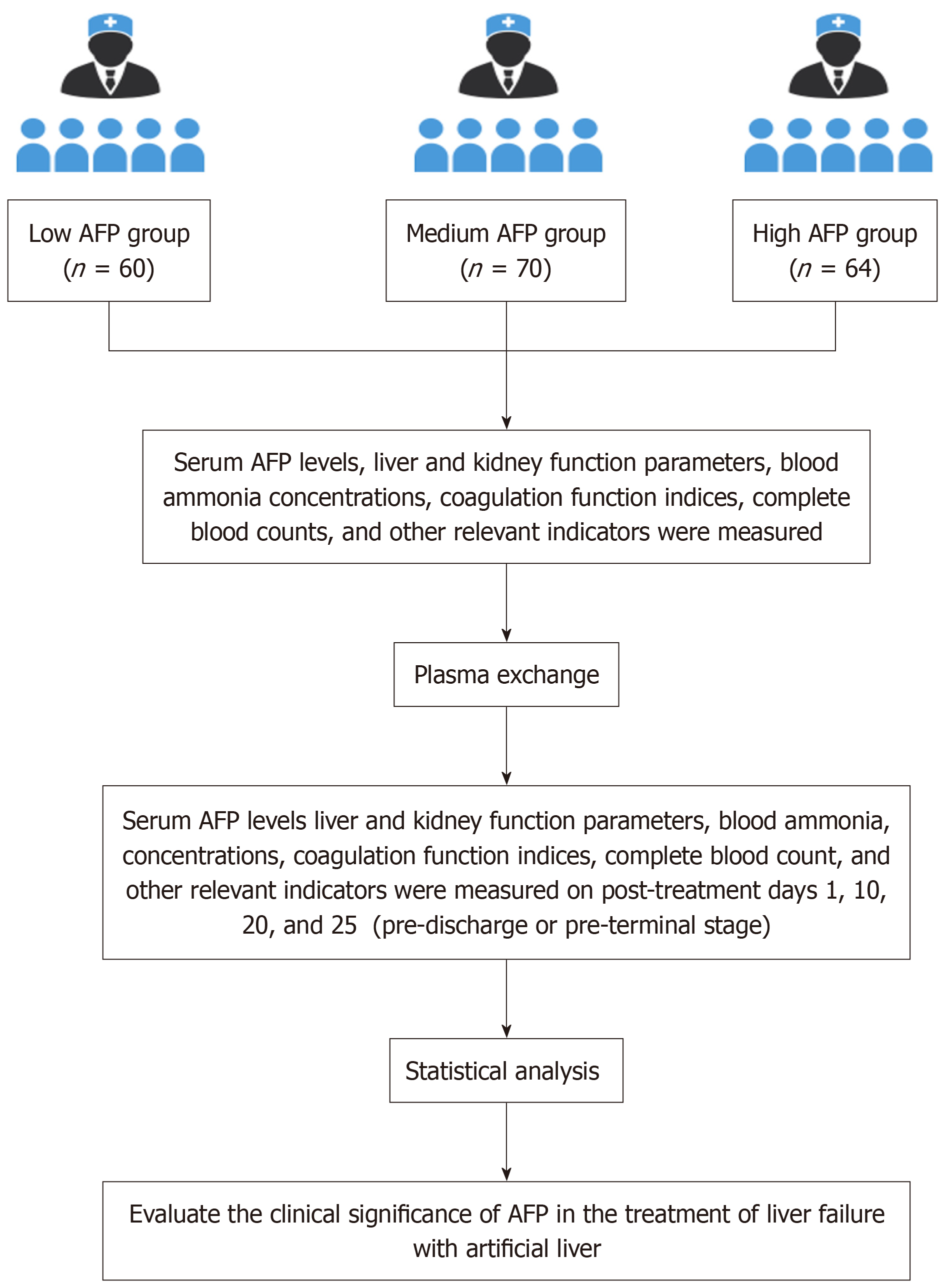
Figure 1 Retrospective study design flowchart.
This figure outlines the design of a retrospective study investigating the clinical significance of alpha-fetoprotein (AFP) in the treatment of liver failure with artificial liver. Patients were stratified into three groups based on serum AFP levels: Low AFP group (n = 60), medium AFP group (n = 70), and high AFP group (n = 64). Baseline measurements included serum AFP levels, liver and kidney function parameters, blood ammonia concentrations, coagulation function indices, complete blood counts, and other relevant indicators. Following plasma exchange treatment, these indicators were reassessed on post-treatment days 1, 10, 20 and 25 points (pre-discharge or pre-terminal stage). AFP: Alpha-fetoprotein.
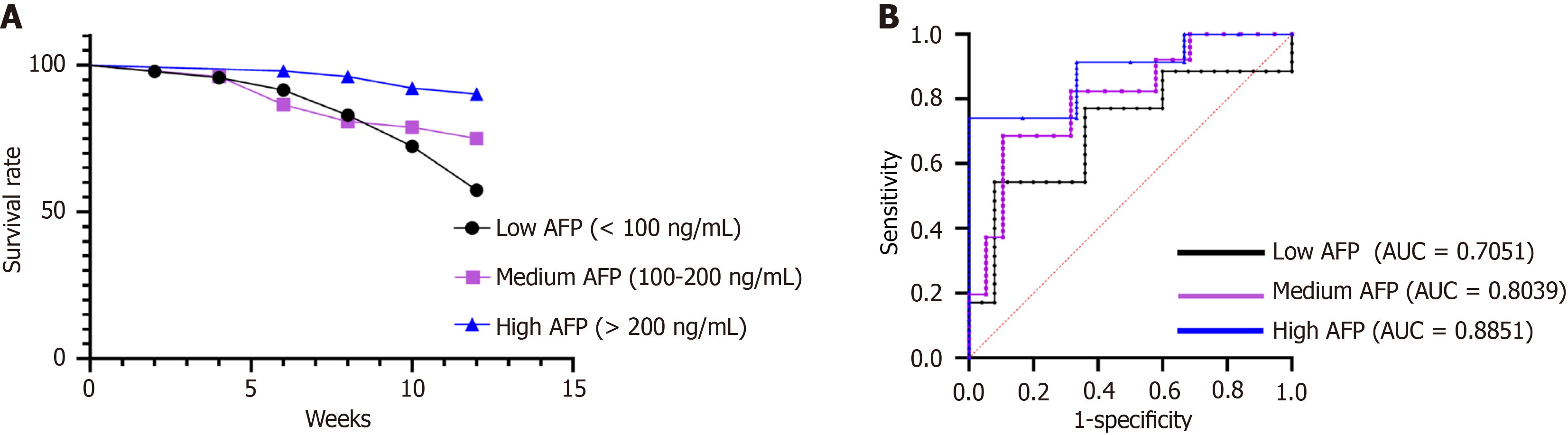
Figure 2 Kaplan-Meier estimates of 3-month survival and areas under the receiver operating characteristic curve calculated for each group at 3 months post-treatment.
A: This figure presents Kaplan-Meier survival curves illustrating the 3-month survival probabilities across the study groups stratified by serum alpha-fetoprotein (AFP) levels (low AFP group, medium AFP group, and high AFP group). The curves visualize differences in survival outcomes among the groups over the 3-month follow-up period; B: This figure displays receiver operating characteristic curves with corresponding areas under the curve values calculated for each group (low AFP group, medium AFP group, and high AFP group) at 3 months post-treatment. The areas under the curve values quantify the diagnostic performance of serum AFP levels in predicting outcomes at the 3-month time point. AFP: Alpha-fetoprotein; AUC: Areas under the curve.
- Citation: Guo WB, Wang LY, Guo XJ, Yang J, Li W, Shen FY, Li YT, Yang JH, Tai WL. Prognostic value of serum alpha-fetoprotein kinetics in liver failure on artificial liver support. World J Gastroenterol 2025; 31(37): 111914
- URL: https://www.wjgnet.com/1007-9327/full/v31/i37/111914.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i37.111914