©The Author(s) 2025.
World J Gastroenterol. Sep 21, 2025; 31(35): 109687
Published online Sep 21, 2025. doi: 10.3748/wjg.v31.i35.109687
Published online Sep 21, 2025. doi: 10.3748/wjg.v31.i35.109687
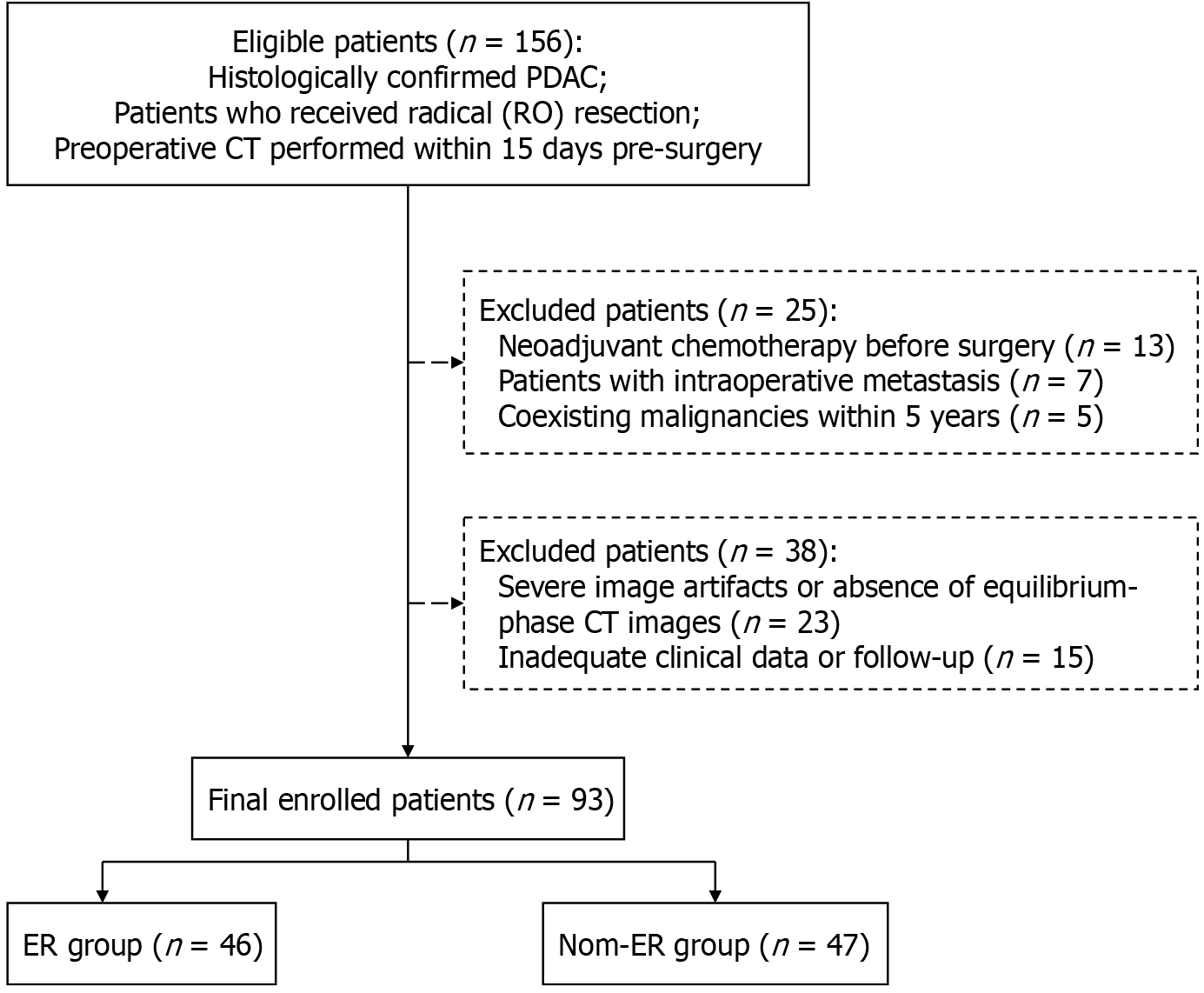
Figure 1 Flow chart.
PDAC: Pancreatic ductal adenocarcinoma; CT: Computed tomography; ER: Early recurrence.
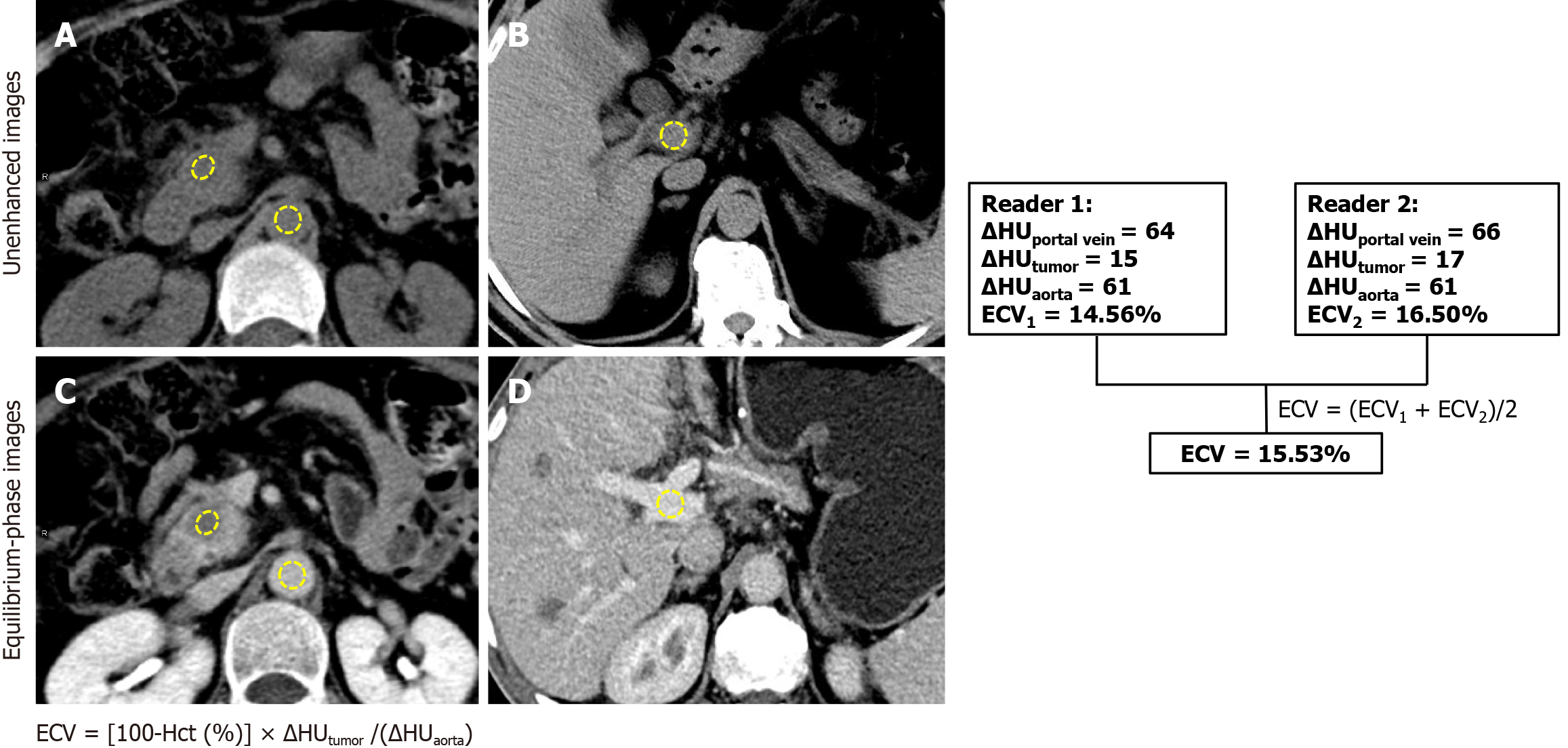
Figure 2 Computed tomography-derived extracellular volume calculation workflow.
The diagram illustrates the extracellular volume (ECV) calculation process in a 61-year-old male with pancreatic ductal adenocarcinoma who underwent radical (R0) resection, with a known hematocrit value of 40.8%. Region of interests were placed on the tumor, portal vein, and abdominal aorta. A and B: In preoperative baseline unenhanced; C and D: Equilibrium phase computed tomography (CT) images. The absolute difference between the ΔHounsfield unit (HU)aorta and ΔHUportal measured < 10 HU, confirming measurement validity. Two independent readers calculated CT-ECV values using the formula shown in the figure, with the final patient ECV (15.53%) derived as the mean of both measurements. ECV: Extracellular volume; Hct: Hematocrit; HU: Hounsfield unit.
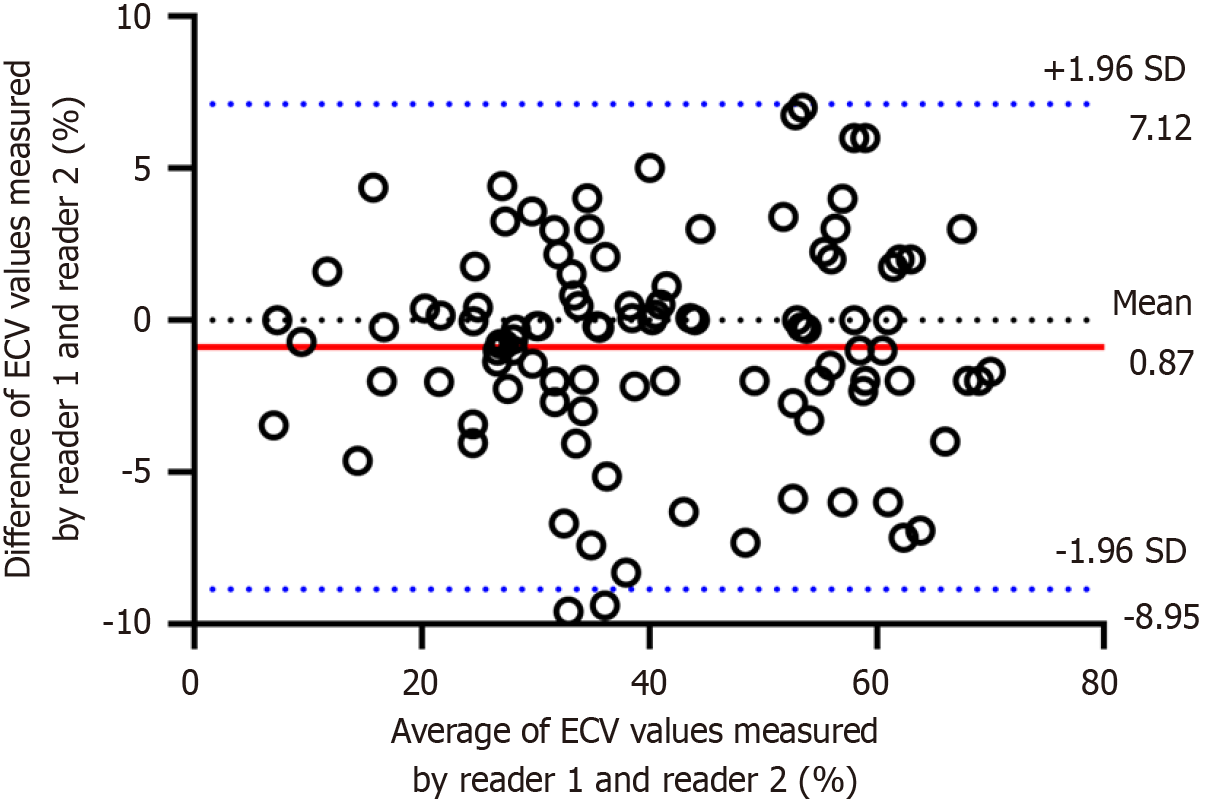
Figure 3 Bland-Altman plots of the computed tomography-derived extracellular volume differences measured by radiologist 1 and radiologist 2.
These Bland-Altman plots showed good agreement. ECV: Extracellular volume.
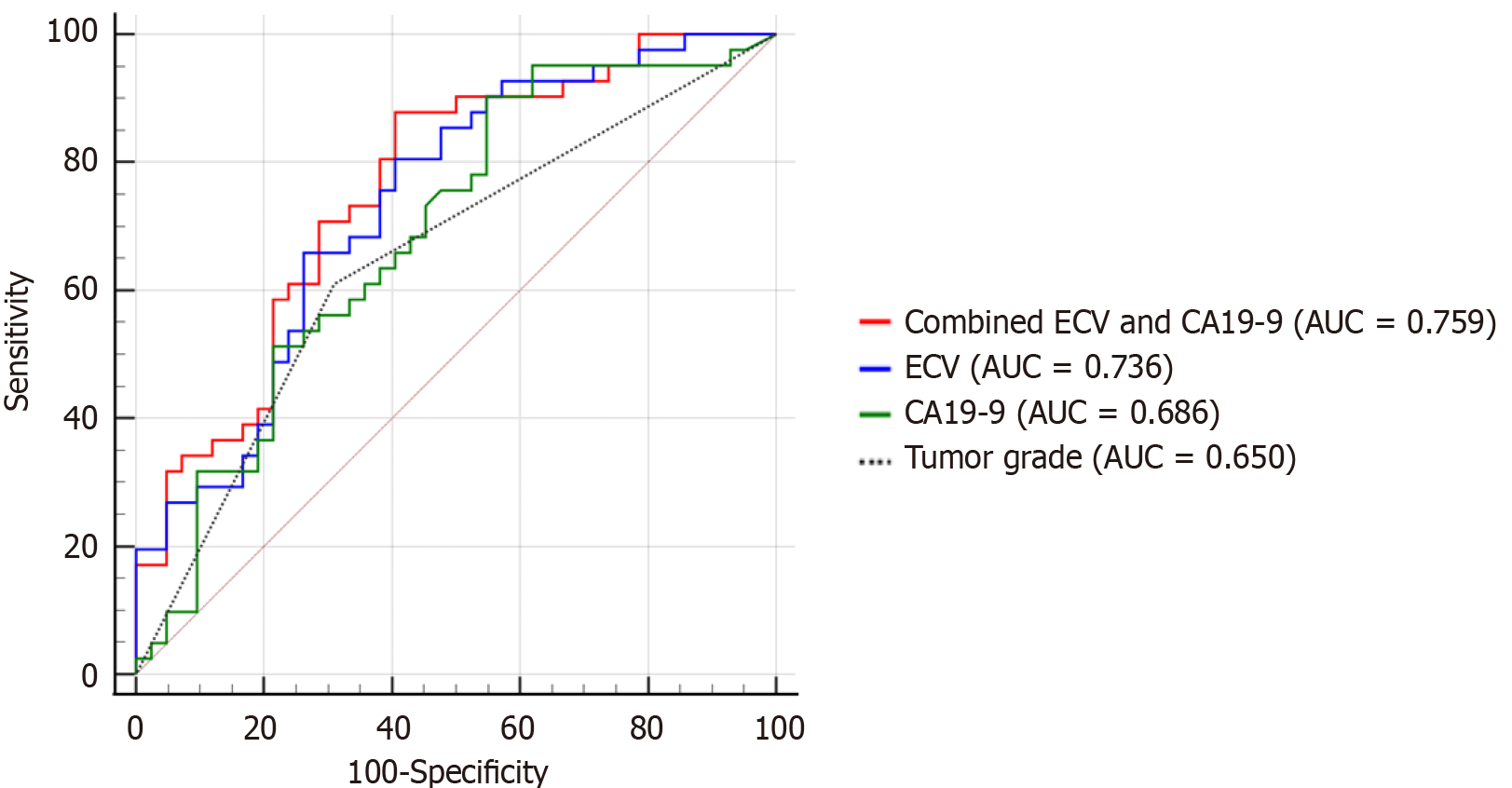
Figure 4 Receiver operating characteristic curve analysis of the computed tomography-derived extracellular volume, carbohydrate antigen 19-9 and tumor grade for predicting early recurrence.
ECV: Extracellular volume; CA19-9: Carbohydrate antigen 19-9; AUC: Area under the curve.
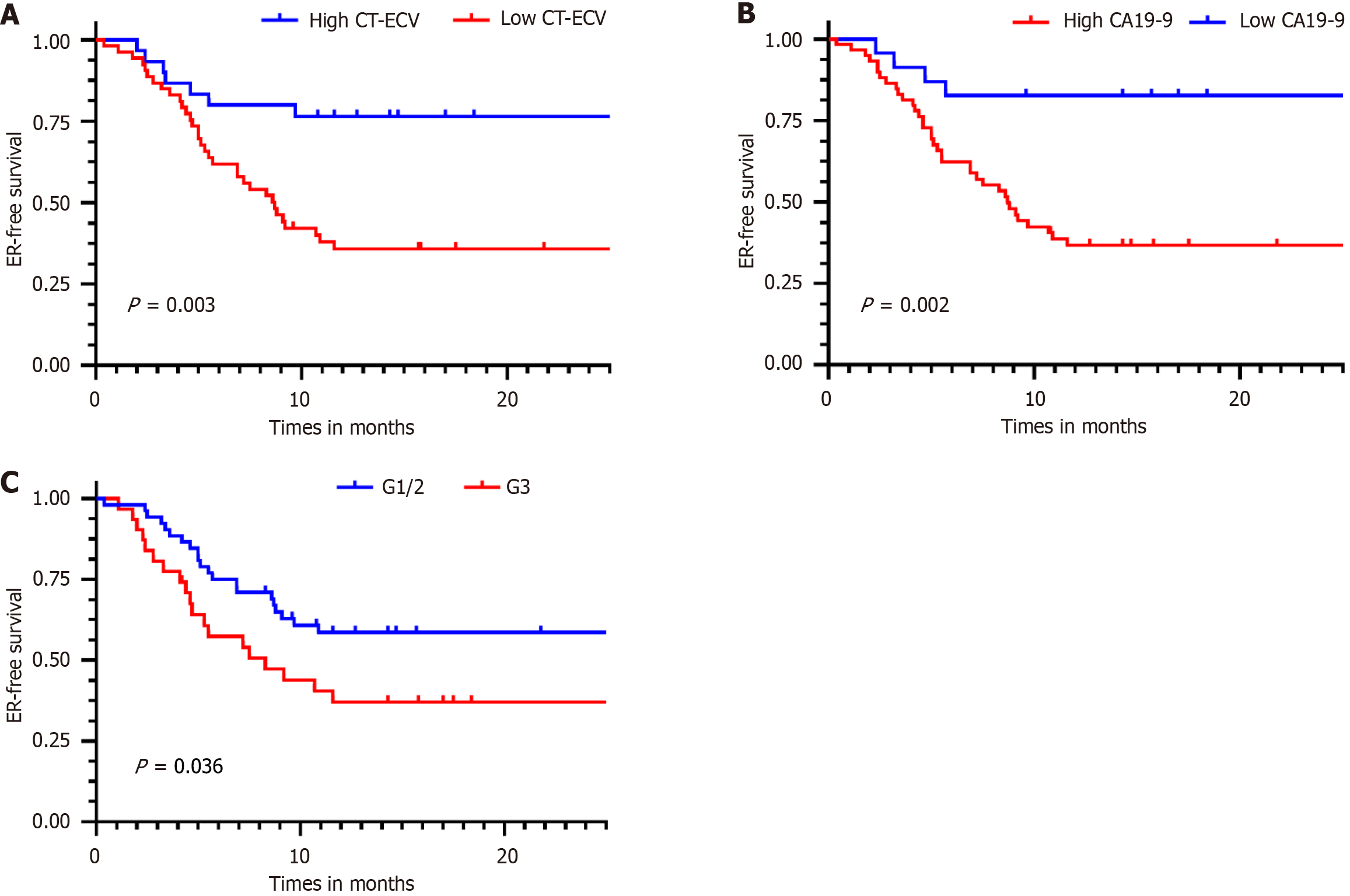
Figure 5 Kaplan-Meier survival curves for early recurrence in patients with pancreatic ductal adenocarcinoma.
A: Stratified by computed tomography-derived extracellular volume; B: Carbohydrate antigen 19-9; C: Tumor grade. CT: Computed tomography; ER: Early recurrence; ECV: Extracellular volume; CA19-9: Carbohydrate antigen 19-9; G1: Well differentiated; G2: Moderately differentiated; G3: Poorly differentiated.
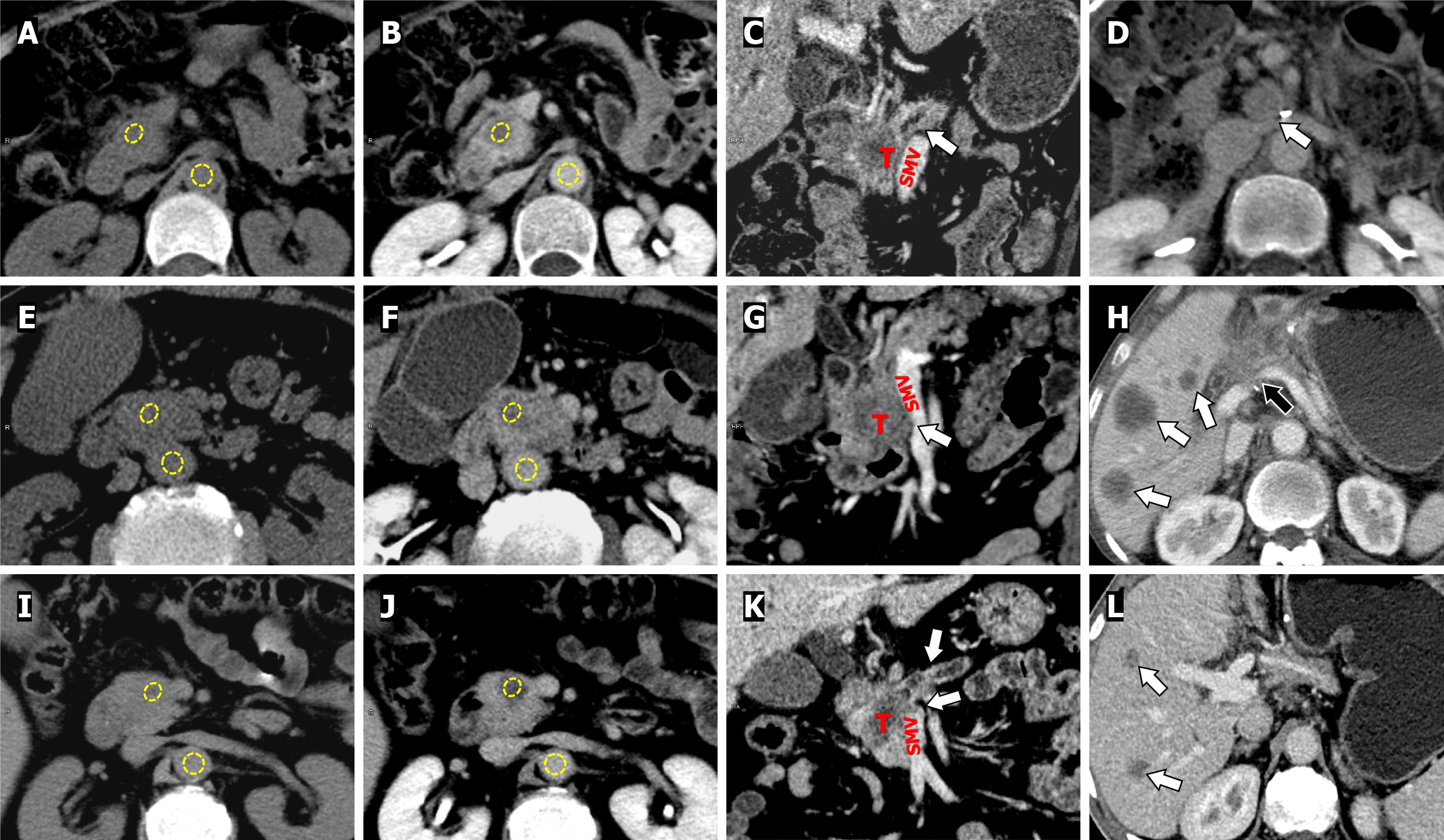
Figure 6 Computed tomography images.
A-D: A 57-year-old female patient with grade G2 (moderately differentiated) pancreatic ductal adenocarcinoma (PDAC) without early recurrence (ER). Axial unenhanced (A) and equilibrium-phase (B) images show regions of interest (ROIs) (in yellow) placed on tumor in the pancreatic head and the aorta. Coronal reconstructed image in portal venous phase shows the dilation of the main pancreatic duct (arrow), and the fat plane between the tumor (T) and superior mesenteric vein (SMV) is clear (C). The CT-derived extracellular volume (ECV) was 37.17%. No tumor recurrence was found in the surgical bed (arrow) after radical resection on follow-up CT (D); E-H: A 70-year-old male patient with grade G3 (poorly differentiated) PDAC with ER. The same ROIs placement demonstration (E and F). Coronal reconstructed image in portal venous phase (G) shows the tumor (T) invading the duodenum (arrow), and interfacing with the SMV without luminal narrowing (arrow). The CT-derived ECV was 28.12%. Tumor local recurrence (black arrow) and multiple hepatic metastases (arrow) occurred 8.3 months after radical resection on postoperative follow-up CT (H); I-L: A 61-year-old male patient with grade G3 (poorly differentiated) PDAC with ER. The same ROIs placement demonstration (I and J). Coronal reconstructed image in portal venous phase shows the pancreatic parenchymal atrophy with main pancreatic duct dilation (arrow), and tumor (T) invade the confluence of portal-SMV (arrow). The CT-derived ECV fraction was 15.53% (K). Hepatic metastasis (arrow) occurred 10.9 months after radical resection on follow-up CT (L). T: Tumor; SMV: Superior mesenteric vein.
- Citation: Zhang ZW, Liu HT, Zhou ZH, Liao HF, Zhang LL, Li YM, Liang HW. Preoperative risk stratification of early recurrence in resected pancreatic ductal adenocarcinoma: Novel equilibrium-phase-computed tomography biomarker of extracellular volume. World J Gastroenterol 2025; 31(35): 109687
- URL: https://www.wjgnet.com/1007-9327/full/v31/i35/109687.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i35.109687