©The Author(s) 2025.
World J Gastroenterol. Sep 7, 2025; 31(33): 108872
Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.108872
Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.108872
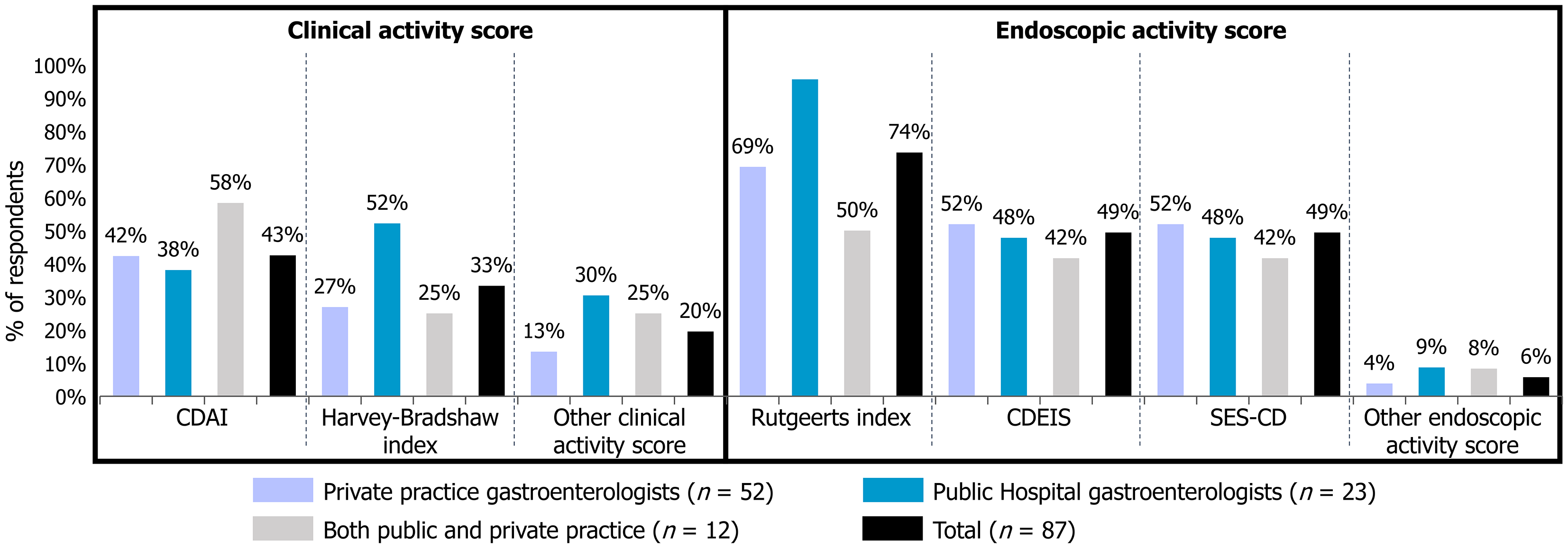
Figure 1 Scores used to assess clinical and endoscopic activity of Crohn’s disease.
Distribution of scores used by respondents to evaluate clinical and endoscopic activity in Crohn’s disease. Multiple answers were allowed to the question: “What score(s) do you use to assess the clinical and endoscopic activity of Crohn’s disease?” (multiple-choice question). Clinical activity scores assess patient symptoms, well-being, and functional status to guide treatment and monitor disease control: Harvey-Bradshaw index: Simplified clinical index based on general well-being, abdominal pain, number of liquid stools, abdominal mass, and extra-intestinal complications; Crohn’s disease activity index: Comprehensive index incorporating stool frequency, abdominal pain, general well-being, hematocrit, weight, use of anti-diarrheal medication, abdominal mass, and complications. Endoscopic activity scores assess mucosal inflammation and healing via endoscopic examination: Simple endoscopic score for Crohn’s disease: Quantifies ulcers, extent of ulcerated surface, affected surface, and narrowing in ileum and colon; Crohn’s disease endoscopic index of severity: Complex index evaluating deep ulceration, surface involvement, ulcerated surface, and stenosis in various bowel segments; Rutgeerts score: Validated for assessing postoperative recurrence after ileocolonic resection; Its application outside postoperative settings is not validated and should be interpreted with caution. CDAI: Crohn’s disease activity index; CDEIS: Crohn’s disease endoscopic index of severity; SES-CD: Simple endoscopic score for Crohn’s disease.
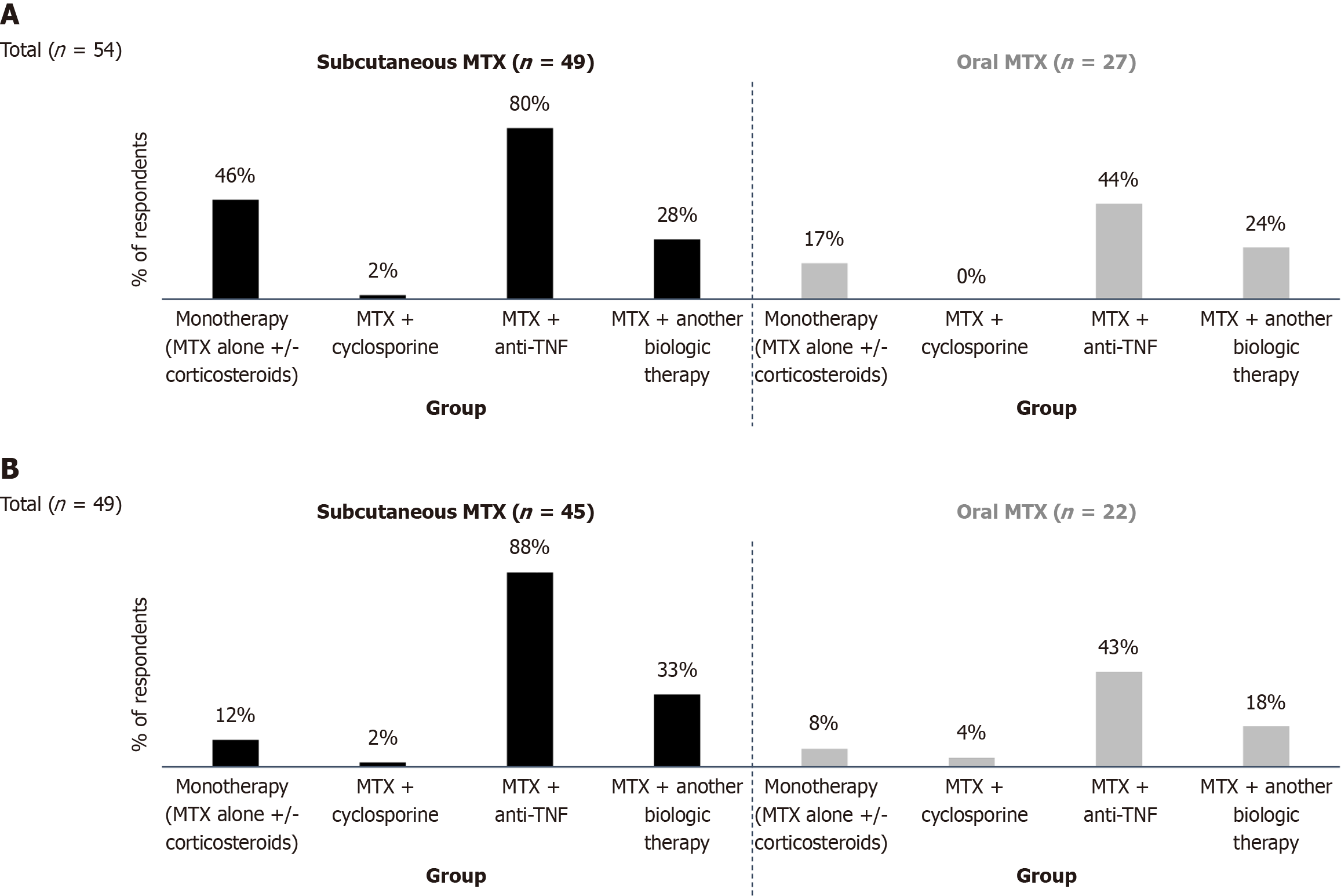
Figure 2 Methotrexate formulations used in Crohn’s disease.
A: Distribution of methotrexate formulations prescribed for mild to moderate Crohn’s disease (CD); B: Formulations used for severe CD. Respondents answered the question: “Do you use methotrexate in oral or subcutaneous form to treat your patients with mild to moderate/severe forms of CD?”. Results show preferences for oral vs subcutaneous formulations in different disease severities. Multiple answers were allowed. Mild CD: CD activity index (CDAI) score between 150 and 220, Harvey-Bradshaw index (HBI) score between 4 and 8. Patients were typically managed in an outpatient setting, exhibited no significant dietary impairment, had weight loss < 10%, and showed no clinical signs of obstruction, fever, dehydration, abdominal mass, or abdominal tenderness. C-reactive protein (CRP) levels were generally elevated above the upper limit of normal; Moderate CD: CDAI score between 220 and 450 or an HBI score between 8 and 12. These patients presented with intermittent vomiting or weight loss > 10%, had inadequate response to minimal treatment for flares, or a tender abdominal mass. There were no overt signs of bowel obstruction. CRP levels were typically elevated above the normal range; Severe CD: CDAI score > 450, HBI > 12, cachexia (body mass index < 18 kg/m2) or obvious obstruction or abscess, persistent symptoms despite intensive treatment, elevated CRP. MTX: Methotrexate; TNF: Tumor necrosis factor.
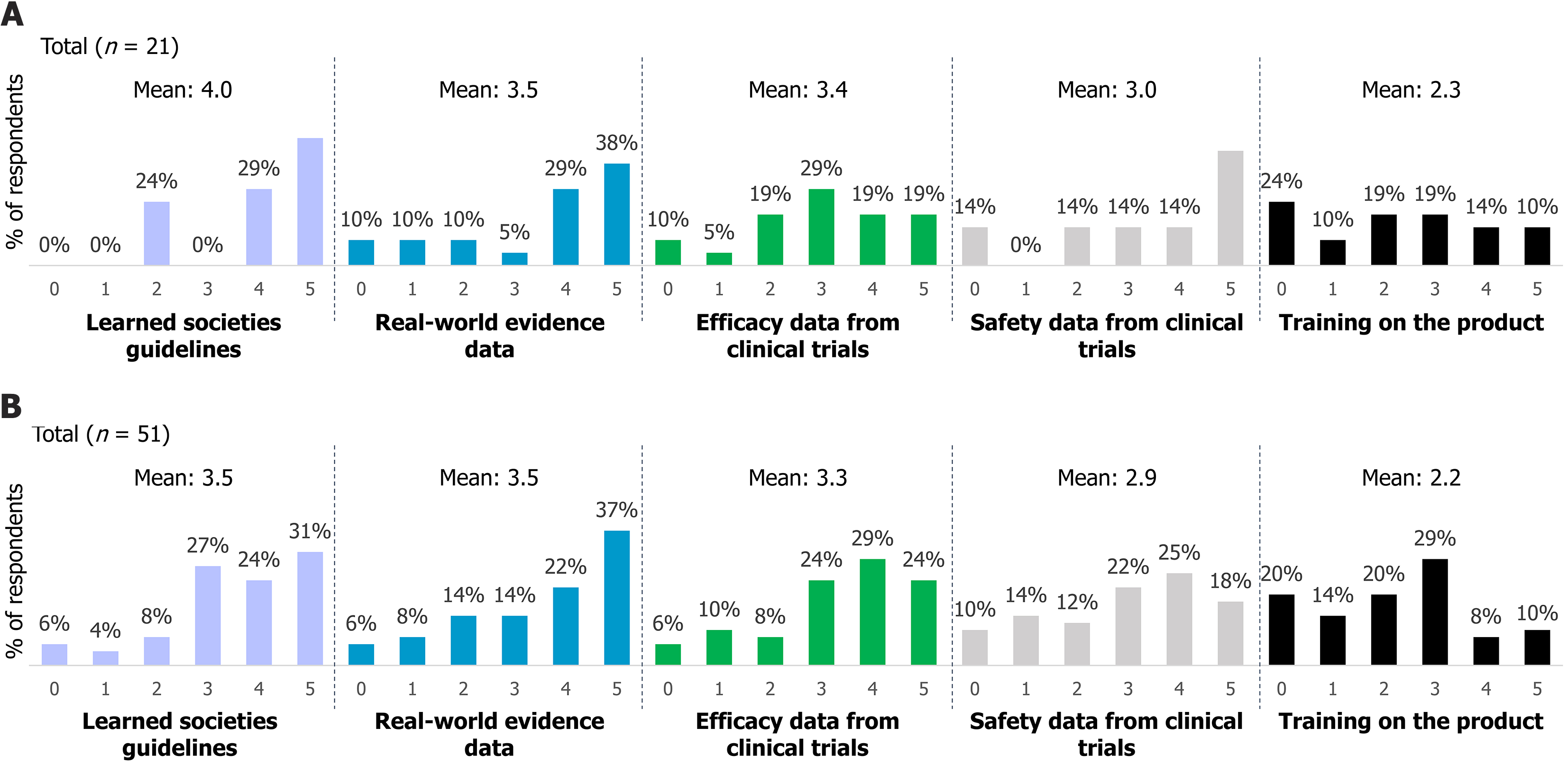
Figure 3 Key factors influencing the practical use of injectable methotrexate in Crohn’s disease management.
A: Responses from methotrexate (MTX) non-prescribers; B: Responses from MTX prescribers. The question posed was: “When the prescription of injectable MTX is medically justified, what additional elements would you need to use this product (on a scale from 0 = unnecessary to 5 = essential)?”. Results illustrate clinicians’ perceived practical requirements to facilitate the use of injectable MTX, such as patient education, administrative support, or enhanced clinical guidelines.
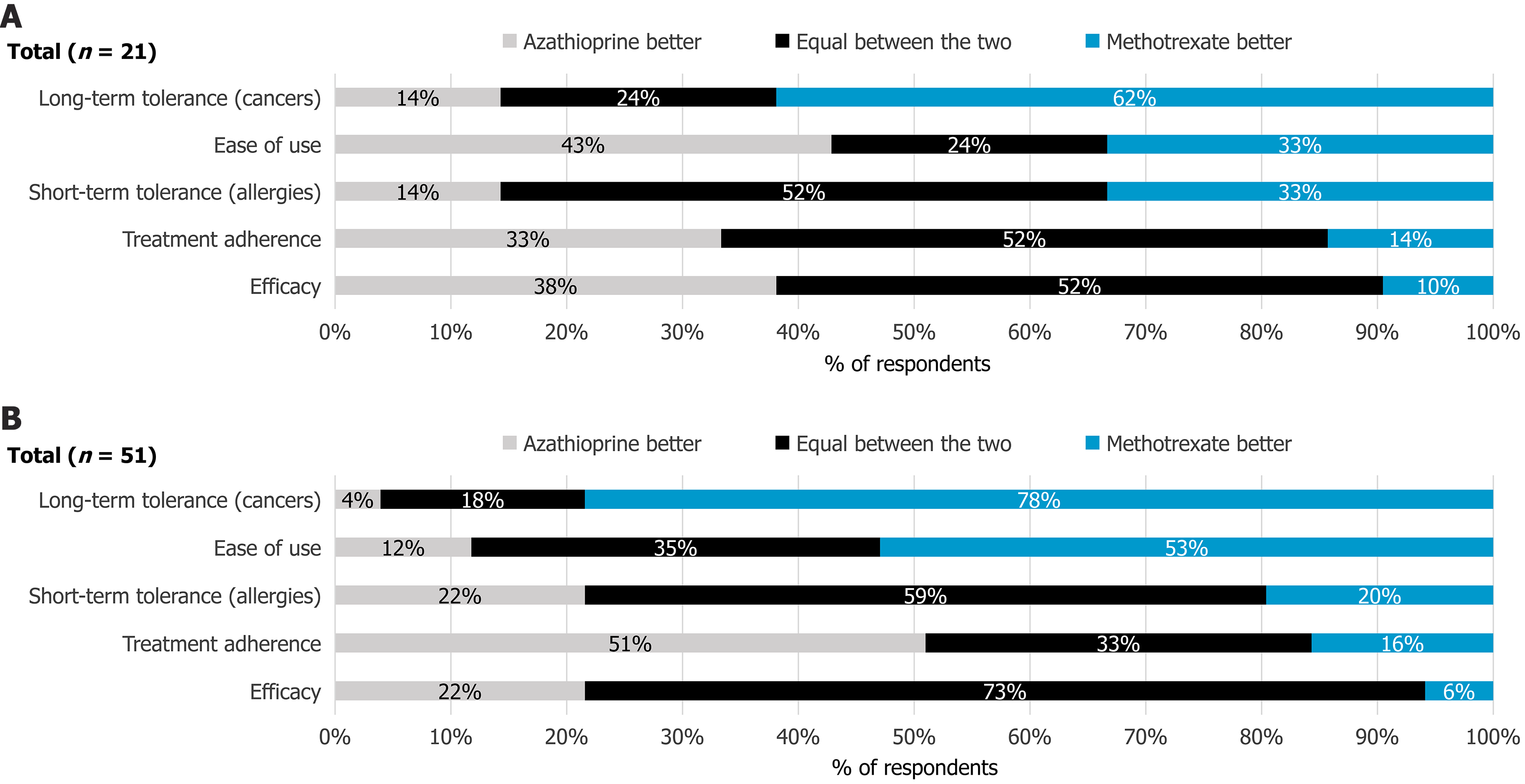
Figure 4 Clinicians’ comparative perception of methotrexate vs azathioprine for Crohn’s disease treatment.
A: Perceptions according to methotrexate (MTX) non-prescribers; B: Perceptions according to MTX prescribers. The question posed was: "How do you rate methotrexate compared with azathioprine in the treatment of Crohn’s disease?" (Single answer question). Respondents compared MTX and azathioprine across dimensions such as efficacy, adherence, short-term and long-term tolerance, and ease of use.
- Citation: Bonnaud G, Becker J, Chebbah M, Courbeyrette A, Faure P. Methotrexate in the management of Crohn’s disease: A practice survey of gastroenterologists in France. World J Gastroenterol 2025; 31(33): 108872
- URL: https://www.wjgnet.com/1007-9327/full/v31/i33/108872.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i33.108872