©The Author(s) 2025.
World J Gastroenterol. Sep 7, 2025; 31(33): 105466
Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.105466
Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.105466
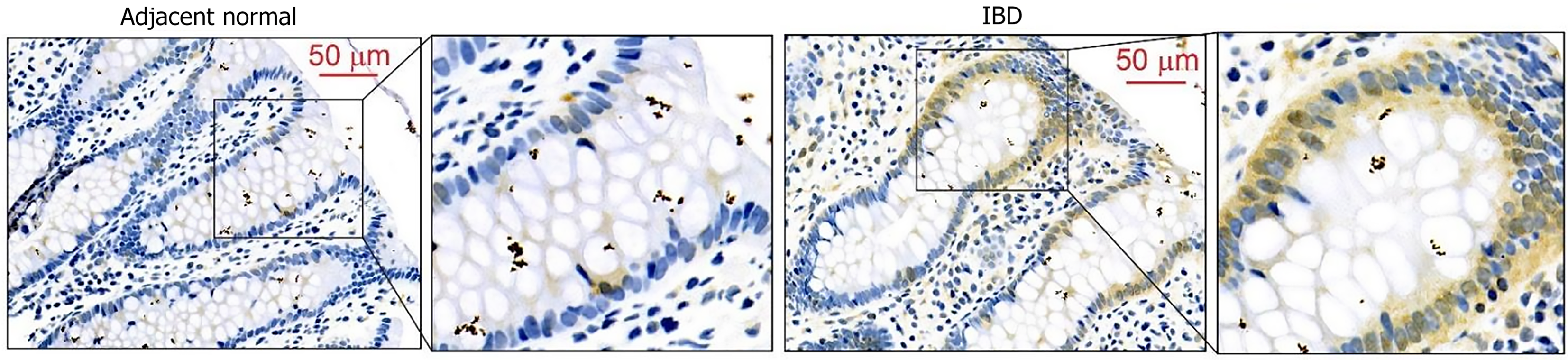
Figure 1 Expression of activated focal adhesion kinase is upregulated in inflammatory bowel disease patients.
Biopsy from inflammatory bowel disease patient’s colon were subjected to immunohistochemistry using an antibody against phosphorylated focal adhesion kinase Tyr397 and images were captured using Nikon T-20 Light microscope. Representative images showing increased focal adhesion kinase phosphorylated at tyrosine 397 in inflammatory bowel disease patients vs adjacent normal (n = 4 individual slides/group). IBD: Inflammatory bowel disease.
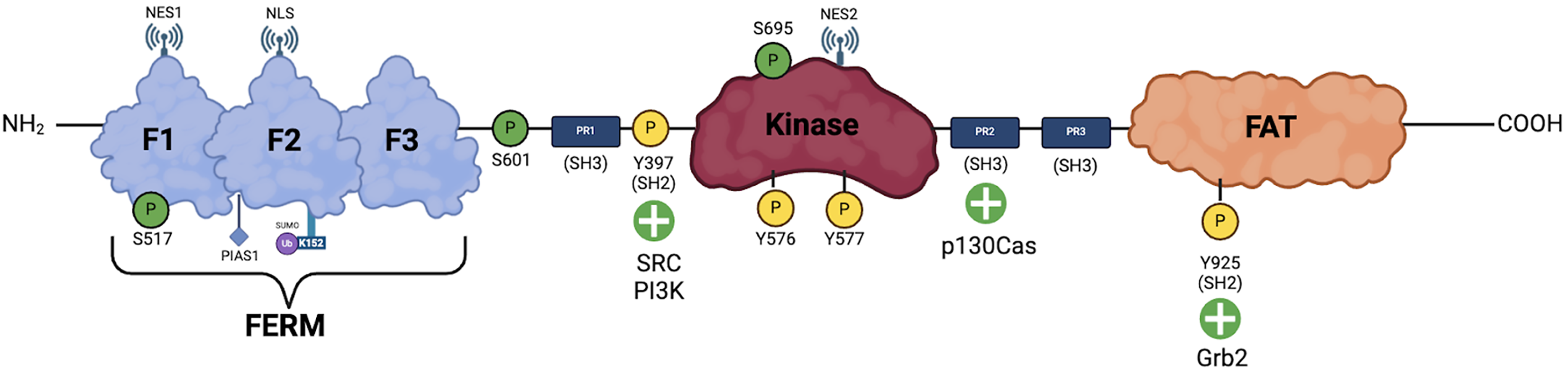
Figure 2 Focal adhesion kinase phosphorylation.
The figure illustrates the phosphorylation of focal adhesion kinase and its interactions with various proteins, highlighting the regulatory mechanisms that govern focal adhesion kinase activation and signaling. Green “P” icons represent the various serine residues that undergo protein kinase B 1 (Akt1)-mediated phosphorylation, yellow “P” icons represent the various tyrosine residues that undergo phosphorylation by various mechanisms. NES: Nuclear export signal; NLS: Nuclear localization signals; PIAS1: Protein inhibitor of activated Stat1; Ub: Ubiquitin; SUMO: Small ubiquitin-related modifier; PR: Progesterone receptor; SH: Src homology domain; PI3K: Phosphoinositide 3-kinase; FAT: Focal adhesion targeting; Grb2: Growth factor receptor-bound protein 2.
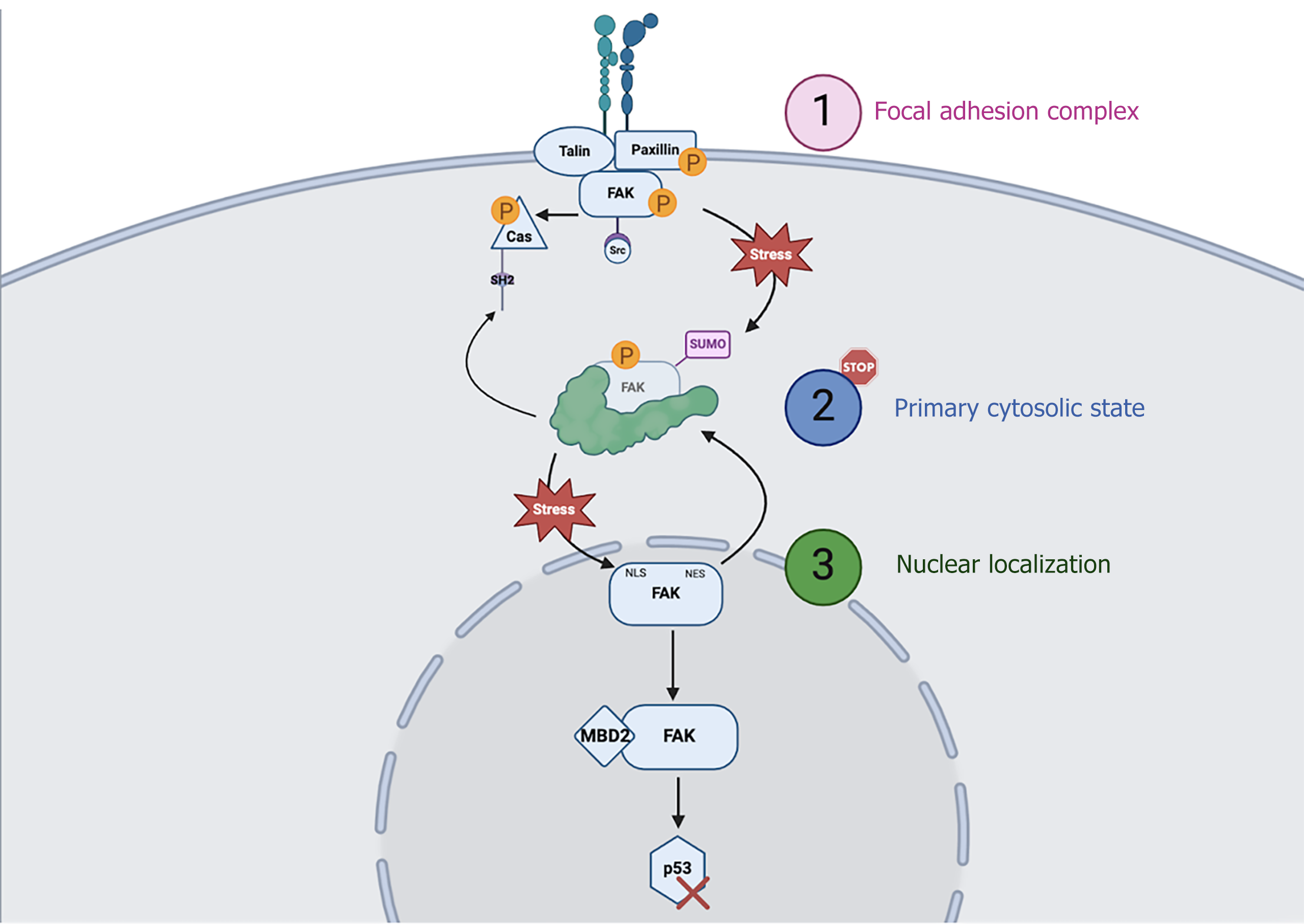
Figure 3 Focal adhesion kinase cycling.
Focal adhesion kinase (FAK) exhibits dynamic cycling between three cellular compartments: (1) The focal adhesion complex at the plasma membrane, where it is activated via autophosphorylation to facilitate integrin signaling and downstream Src and CRISPR associated proteins protein recruitment; (2) The primary cytosolic state, where FAK resides in an inactive or post-translationally modified (e.g., SUMOylated) reservoir, and (3) The nucleus, where stress-induced translocation via the nuclear localization signals triggers FAK nuclear functions, such as binding methyl-CpG-binding domain protein and promoting p53 degradation. FAK’s shuttling is tightly regulated, and its return to the cytosol is mediated by nuclear export signals. MBD2: Methyl-CpG-binding domain protein; FAK: Focal adhesion kinase; Cas: CRISPR associated proteins; SH: Src homology domain; SUMO: Small ubiquitin-related modifier; NES: Nuclear export signal; NLS: Nuclear localization signals.
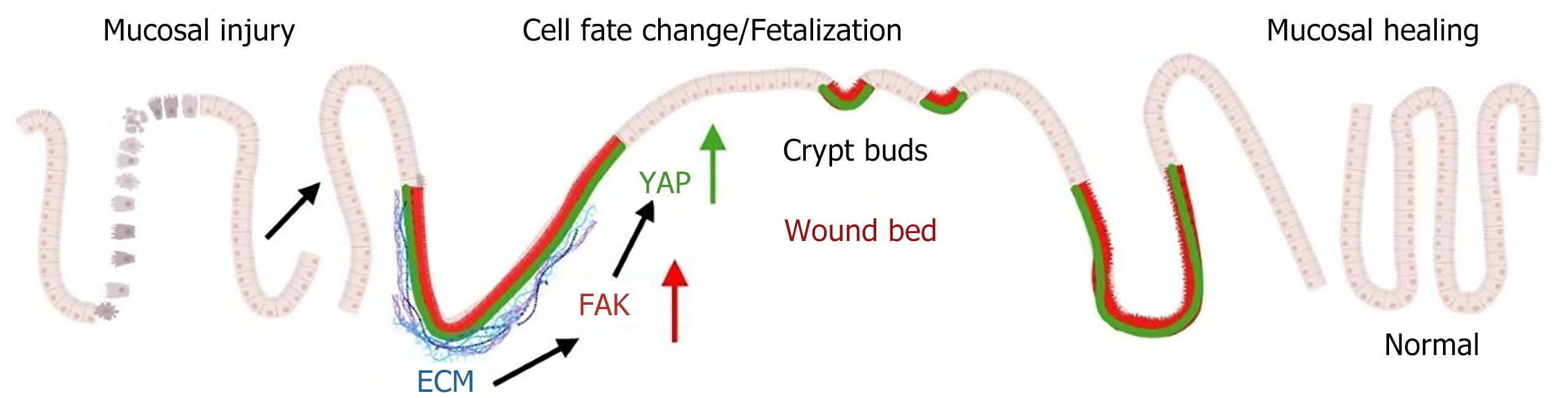
Figure 4 Schematic overview of colonic epithelial wound healing.
Injury induces upregulated expression of extracellular matrix components, including β1 integrin and collagen, which form a supportive scaffold for epithelial cell migration and adhesion. β1 integrin engages with the extracellular matrix to activate focal adhesion kinase. Through its interaction with Src kinase, focal adhesion kinase subsequently activates Yes-associated protein. The activation of Yes-associated protein promotes epithelial cell proliferation, a crucial process for initiating mucosal repair/restitution. ECM: Extracellular matrix; FAK: Focal adhesion kinase; YAP: Yes-associated protein.
- Citation: Malek KK, Kumar B, Ahmad R, Singh A, Basson MD. Focal adhesion kinase: A promising regulator of colitis-associated healing. World J Gastroenterol 2025; 31(33): 105466
- URL: https://www.wjgnet.com/1007-9327/full/v31/i33/105466.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i33.105466