©The Author(s) 2025.
World J Gastroenterol. Aug 7, 2025; 31(29): 109090
Published online Aug 7, 2025. doi: 10.3748/wjg.v31.i29.109090
Published online Aug 7, 2025. doi: 10.3748/wjg.v31.i29.109090
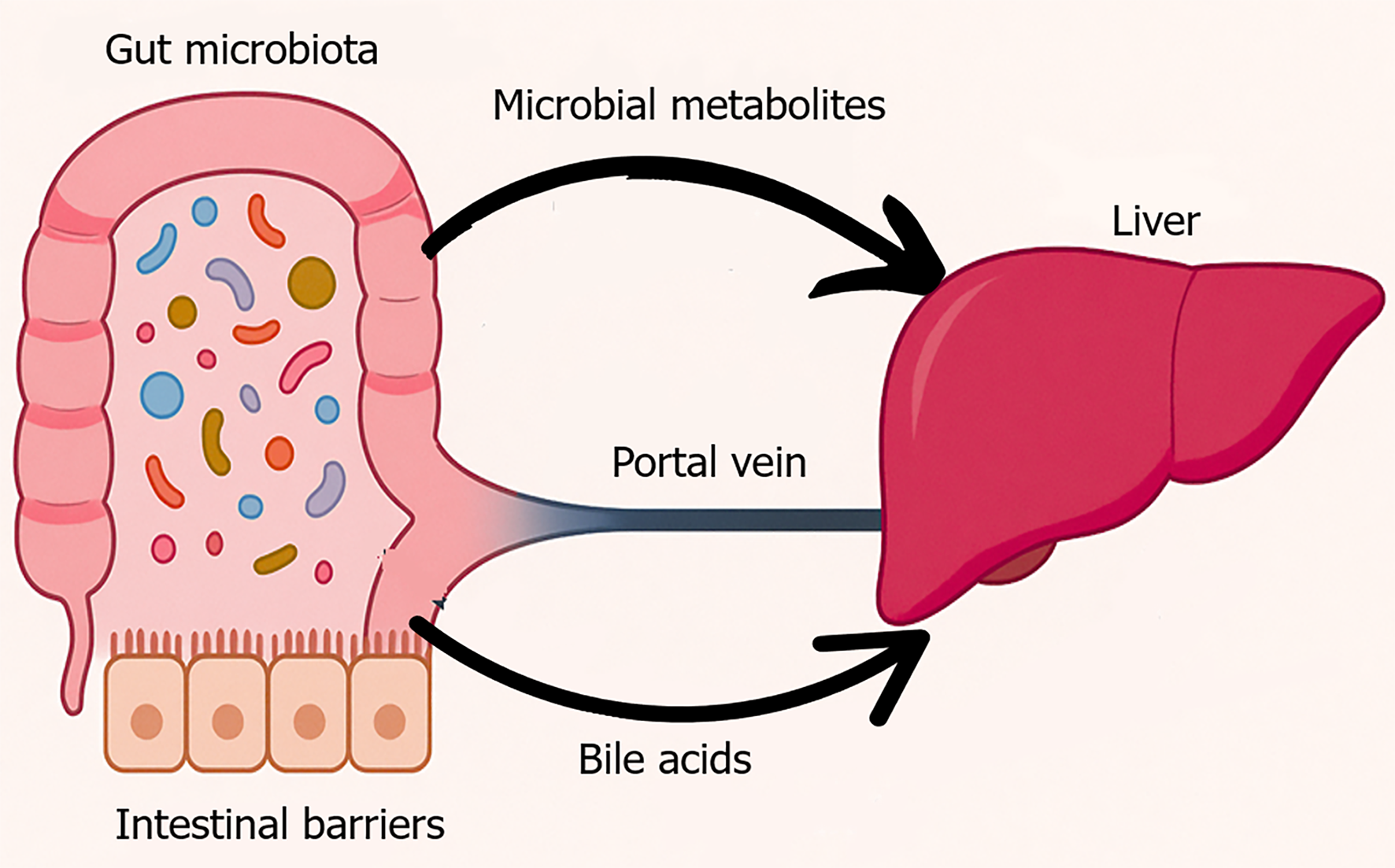
Figure 1 Physiological gut liver axis.
This figure visually represents the anatomical and functional connectivity between the gut and liver, commonly referred to as the gut-liver axis. In a healthy state, a diverse and balanced gut microbiota resides within the intestinal lumen, supported by an intact epithelial barrier. Microbial metabolites such as short-chain fatty acids and secondary bile acids enter the portal circulation and interact with the liver to regulate glucose and lipid metabolism, immune tolerance, and bile acid recycling. This bidirectional communication maintains metabolic and immune homeostasis.
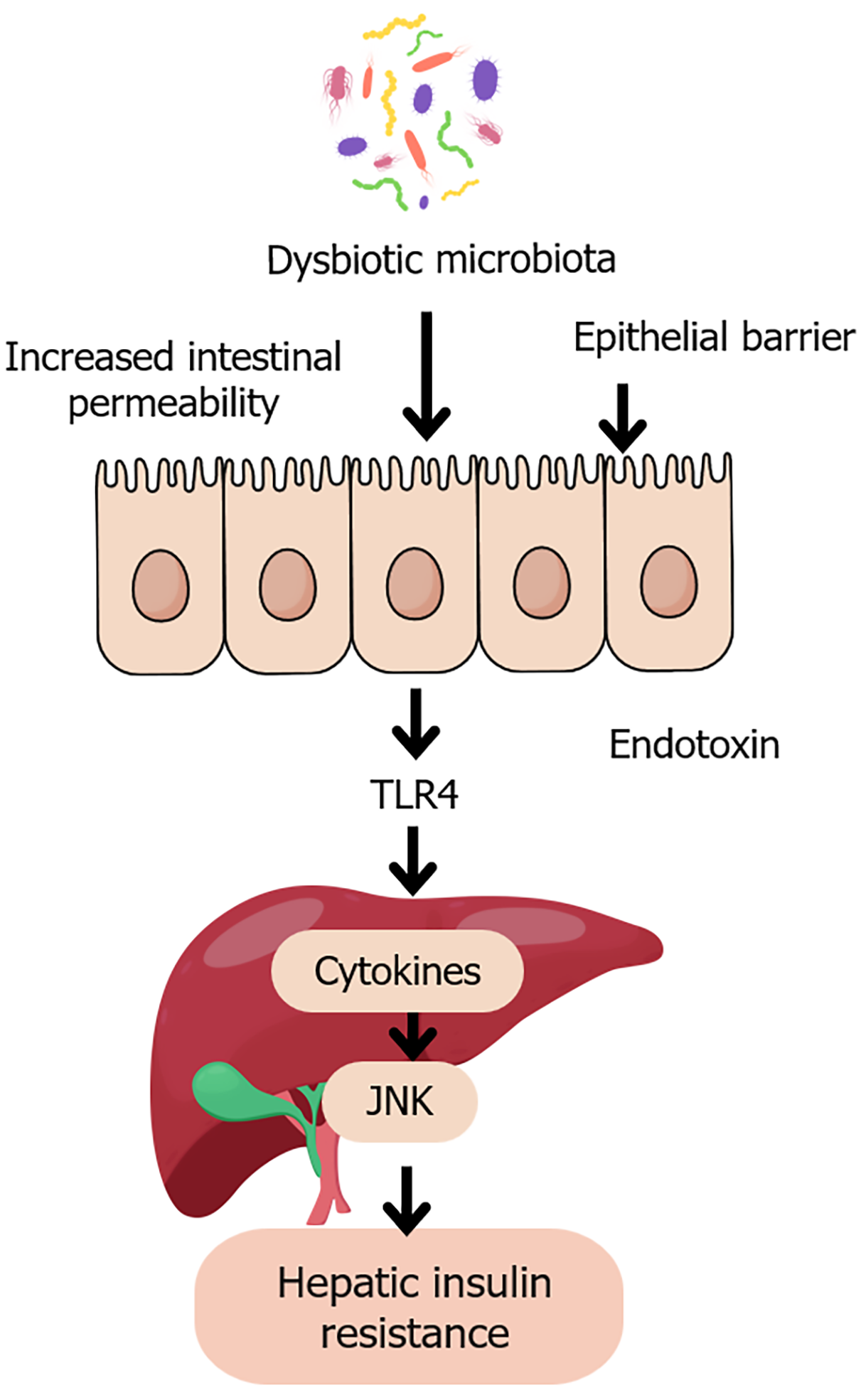
Figure 2 Gut dysbiosis and hepatic insulin resistance.
This figure is a schematic illustration of the pathological pathway linking dysbiotic gut microbiota to hepatic insulin resistance. Disruption of microbial balance leads to increased intestinal permeability and epithelial barrier dysfunction, allowing the translocation of endotoxins such as lipopolysaccharide into the portal circulation. These endotoxins activate toll-like receptor 4 on hepatic Kupffer cells, triggering pro-inflammatory cytokine release and activation of c-Jun N-terminal kinase signaling. This inflammatory cascade impairs insulin receptor signaling, contributing to hepatic insulin resistance, a key feature of type 2 diabetes. JNK: C-Jun N-terminal kinase; TLR4: Toll-like receptor 4.
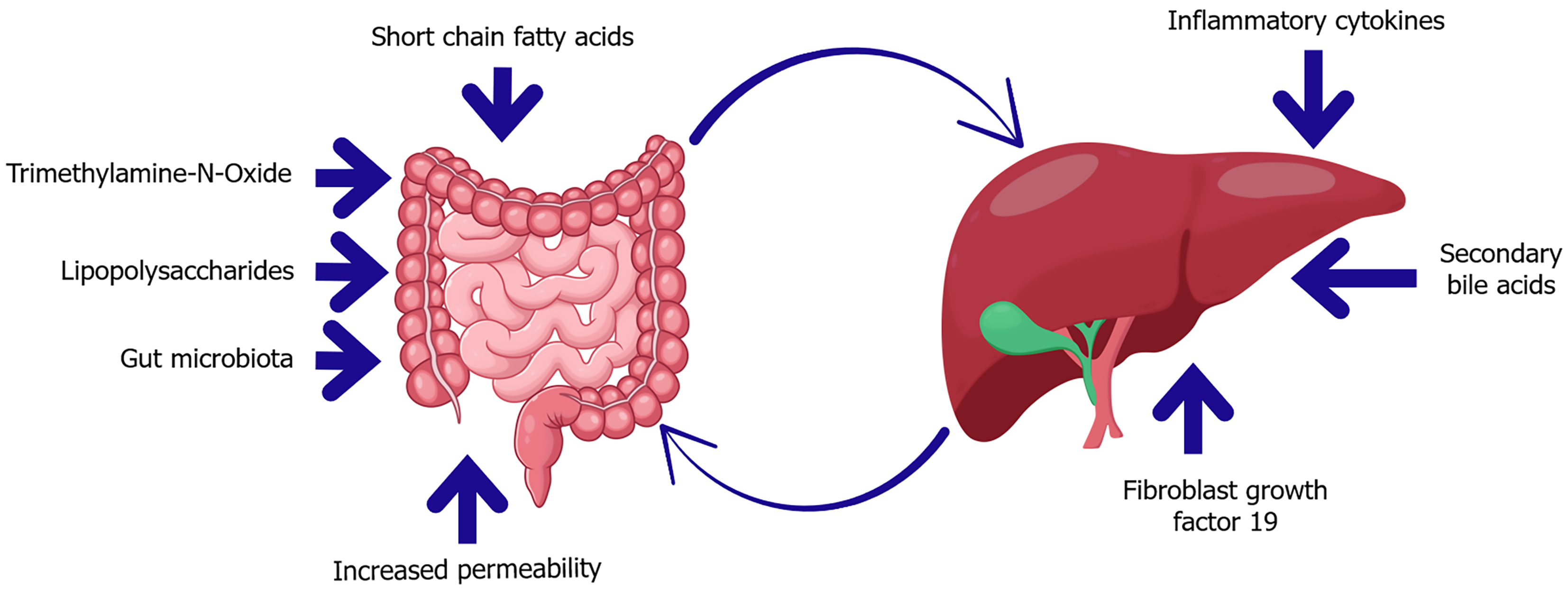
Figure 3 Key mediators of the gut-liver axis in diabetes and insulin resistance.
This diagram highlights bidirectional communication between the gut and liver mediated by microbial and host-derived factors. In dysbiosis, microbial metabolites such as trimethylamine-N-oxide and lipopolysaccharides increase gut permeability, enabling translocation of endotoxins into the portal circulation. These signals promote hepatic inflammation and insulin resistance. Conversely, the liver influences gut homeostasis through the secretion of secondary bile acids and fibroblast growth factor 19, which regulate microbial composition and barrier integrity. Short-chain fatty acids also play a protective role, supporting epithelial health and metabolic balance.
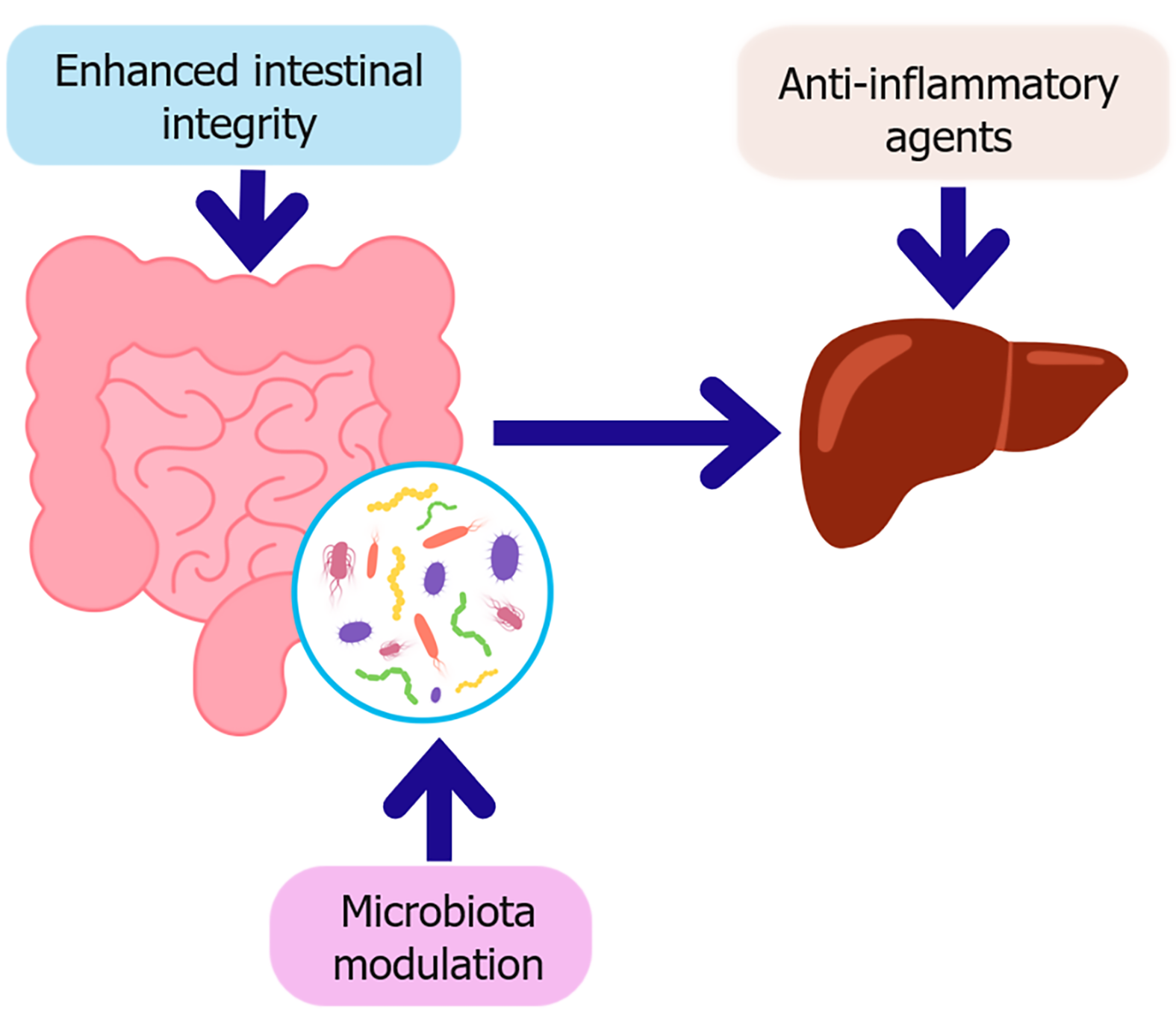
Figure 4 Key therapeutic strategies targeting the gut-liver axis in diabetes.
This schematic illustrates therapeutic strategies aimed at restoring gut-liver axis homeostasis in the context of type 2 diabetes. Microbiota modulation, through probiotics, prebiotics, synbiotics, or fecal microbiota transplantation which enhances intestinal integrity by strengthening the epithelial barrier and promoting beneficial metabolites such as short-chain fatty acids. Improved barrier function reduces microbial translocation, subsequently decreasing hepatic inflammation. In parallel, anti-inflammatory agents directly target hepatic immune activation, contributing to improved insulin sensitivity and metabolic regulation.
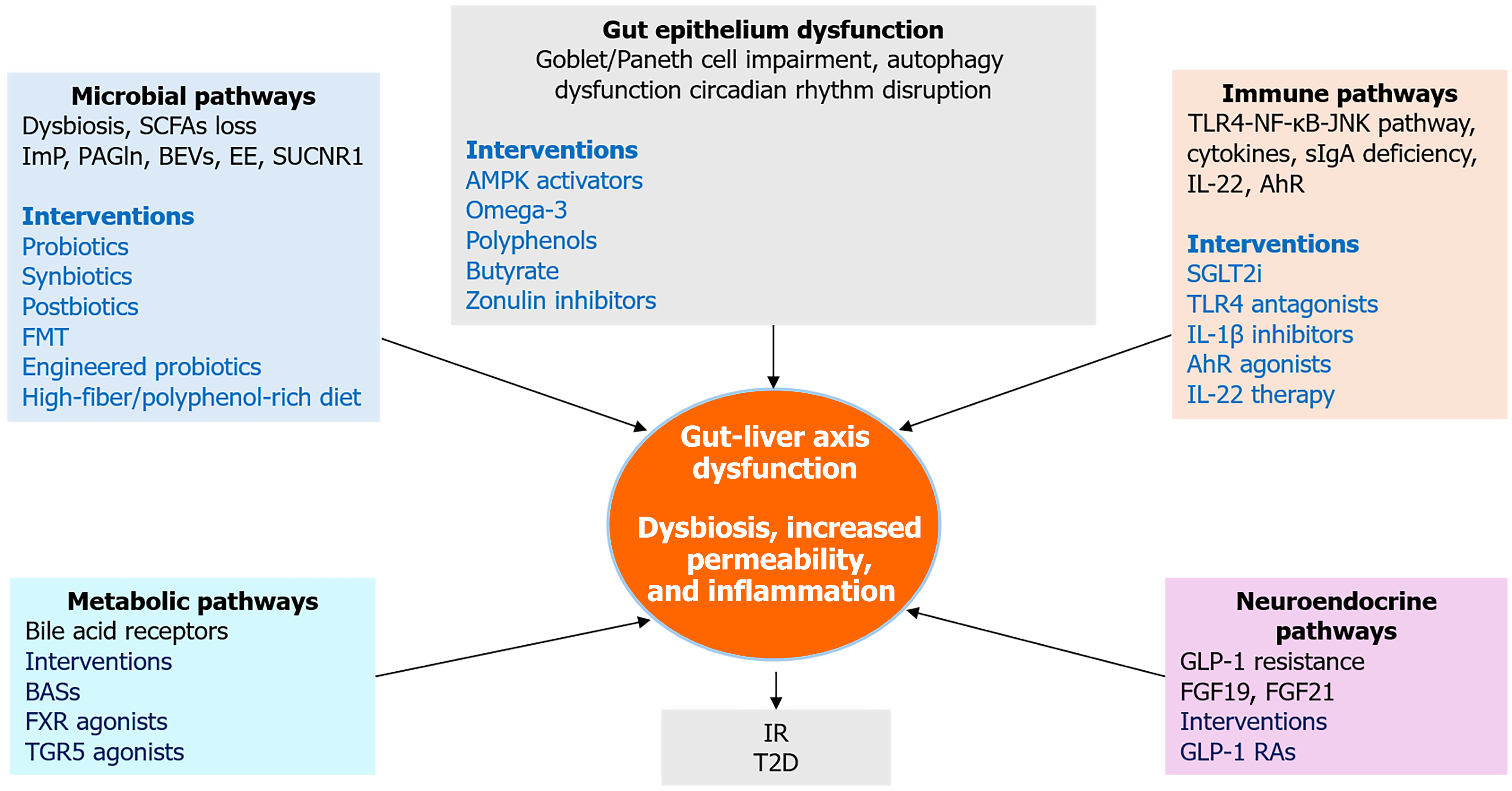
Figure 5 Integrative therapeutic and mechanistic pathways linking gut and liver in type 2 diabetes.
This integrative framework illustrates how five key mechanistic domains, microbial dysbiosis, gut epithelial dysfunction, immune activation, neuroendocrine disruption, and metabolic signaling which converge to drive gut-liver axis dysfunction in type 2 diabetes. Each domain includes representative molecular mediators and therapeutic interventions. The central node highlights dysbiosis, increased permeability, and inflammation as core features linking upstream disruptions to downstream insulin resistance and hepatic steatosis. SCFAs: Short-chain fatty acids; ImP: Imidazole propionate; PAGln: Phenylacetylglutamine; BEVs: Bacterial extracellular vesicles; EE: Endogenous ethanol; FMT: Fecal microbiota transplantation; BASs: Bile acid sequestrants; FXR: Farnesoid X receptor; TGR5: Takeda G protein-coupled receptor 5; AMPK: Adenosine 5’-monophosphate-activated protein kinase; IR: Insulin resistance; T2D: Type 2 diabetes; TLR4: Toll-like receptor 4; JNK: C-Jun N-terminal kinase; NF-κB: Nuclear factor kappa B; sIgA: Secretory immunoglobulin A; IL-1β: Interleukin-1 beta; IL-22: Interleukin-22; AhR: Aryl hydrocarbon receptor; SGLT2i: Sodium-glucose cotransporter-2 inhibitors; FGF19: Fibroblast growth factor 19; FGF21: Fibroblast growth factor 21; GLP-1: Glucagon-like peptide-1; GLP-1 RAs: Glucagon-like peptide-1 receptor agonists.
- Citation: Abdalla MMI. Gut-liver axis in diabetes: Mechanisms and therapeutic opportunities. World J Gastroenterol 2025; 31(29): 109090
- URL: https://www.wjgnet.com/1007-9327/full/v31/i29/109090.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i29.109090