©The Author(s) 2025.
World J Gastroenterol. Jul 28, 2025; 31(28): 108297
Published online Jul 28, 2025. doi: 10.3748/wjg.v31.i28.108297
Published online Jul 28, 2025. doi: 10.3748/wjg.v31.i28.108297
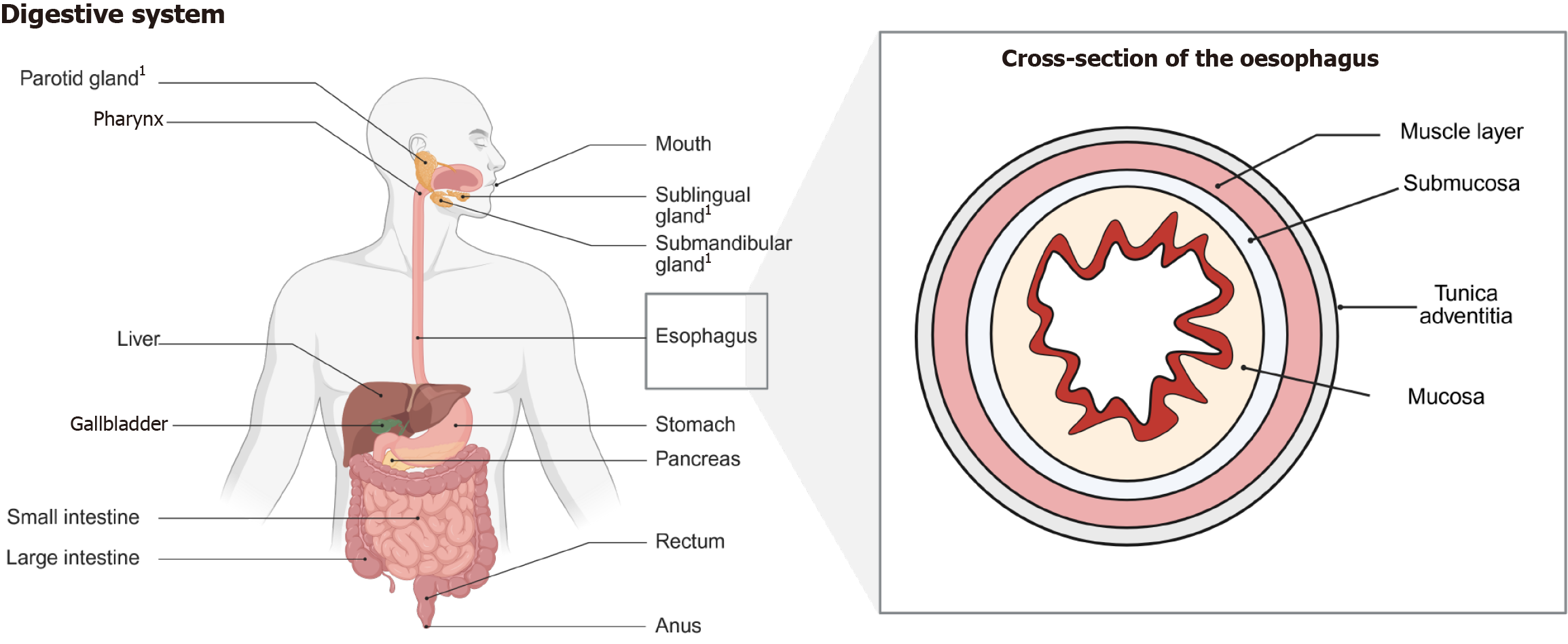
Figure 1 Representation of the digestive system and the oesophagus.
The digestive system is composed of various organs which are playing important roles in the digestion, absorption, excretion, and protection of the human body. The individual organs differ in their composition and corresponding diseases. The structure of the oesophagus is displayed as a cross section. The depiction presents multiple layers, which are important to know due to implications of physiological function as well as staging of malignant diseases (infiltration depth) and its corresponding treatment (i.e., mucosectomy or fill thickness resection); 1Salivary glands.
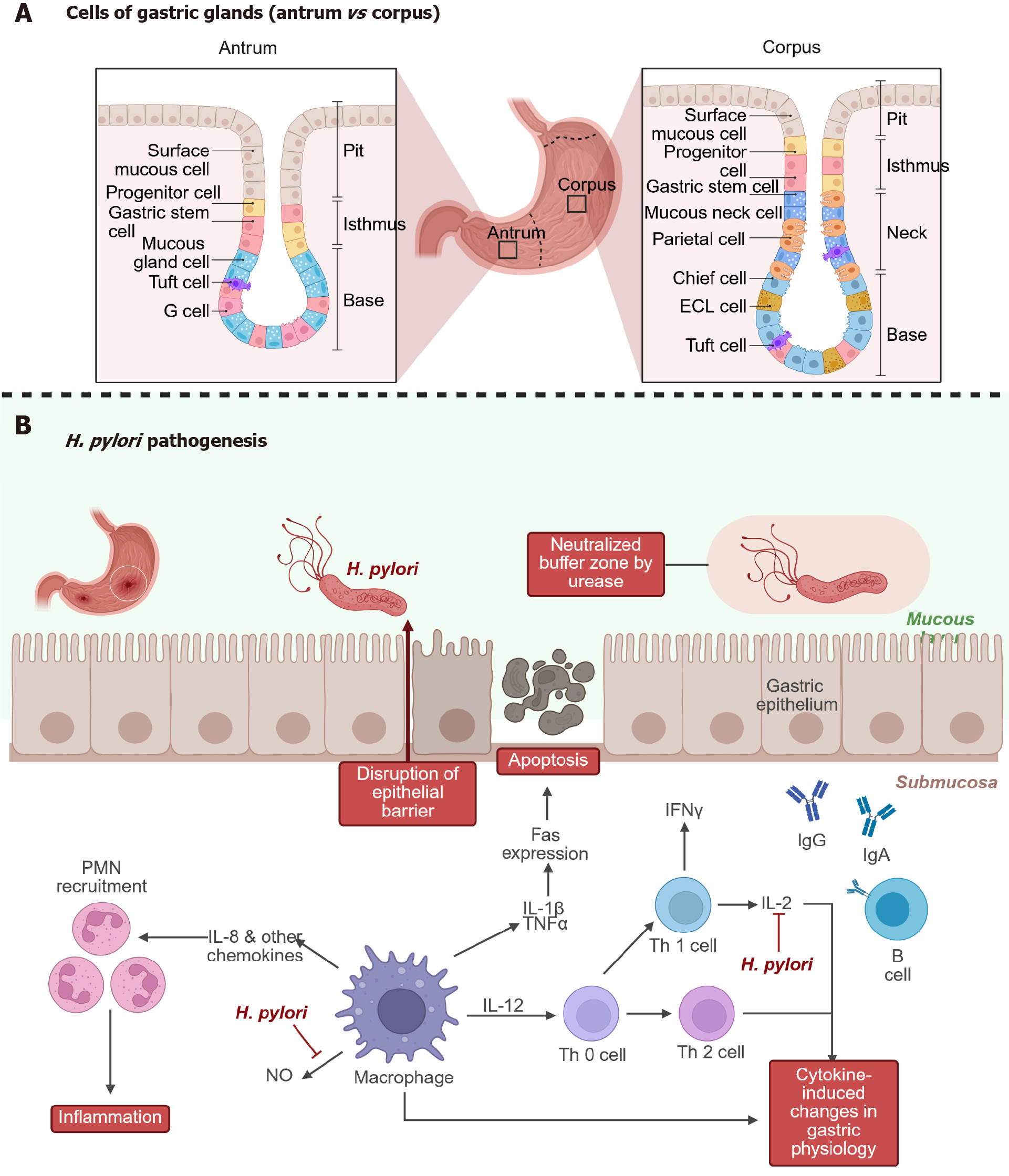
Figure 2 The stomach, its structure and pathogenesis of Helicobacter pylori disease.
A: Comparison between gastric mucosa in the antrum and in the corpus; B: The pathogenesis of Helicobacter pylori infection-Helicobacter pylori reaches the stomach epithelium by swimming through mucus and neutralizing acid with urease. The bacterium adheres to epithelial cells and disrupts the epithelial barrier, leading to apoptosis and tissue damage. It induces interleukin (IL)-8 and other chemokines, which recruit neutrophils and macrophages. Cytokines like IL-1β, tumor necrosis factor-α, and IL-12 promote inflammation. Adaptive immunity is also engaged: T helper cells (Th1/Th2) and B cells produce antibodies (IgG, IgA), though often insufficient to clear the infection. The result is chronic inflammation, immune dysregulation, and cytokine-driven changes in gastric physiology. This may contribute to gastritis, ulcer formation, and even gastric cancer. H. pylori: Helicobacter pylori; PMN: Polymorphonuclear leukocyte; IL: Interleukin; TNF: Tumor necrosis factor; IFN: Interferon; NO: Nitric oxide.
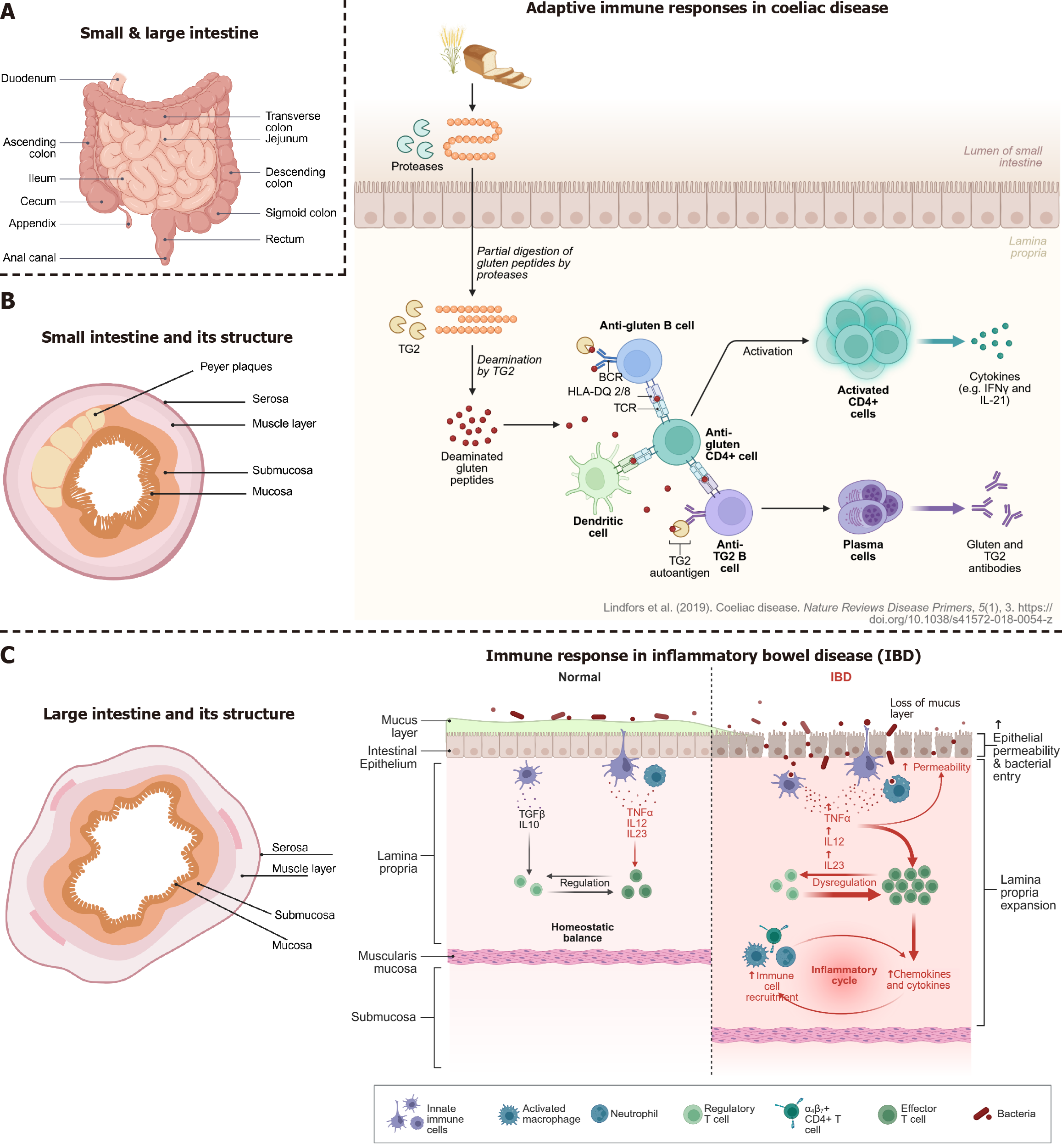
Figure 3 The small and large intestine, their anatomy and diseases overview of intestinal structure and immune responses in coeliac disease and inflammatory bowel disease.
A: Anatomical overview of the small and large intestine; B: Cross-sectional structure of the small intestine, highlighting key layers (left part): Mucosa, submucosa, muscle layer, and serosa, which play roles in barrier function and immune signaling. Peyer’s patches in the small intestine are important sites for immune activation (right part). In coeliac disease, gluten peptides are incompletely digested and deamidated by tissue transglutaminase (TG2), forming neoantigens that are presented to cluster of differentiation 4 + T cells by human leucocyte antigen-DQ2/8 molecules. This leads to T cell activation, B cell maturation, and production of anti-gluten and anti-TG2 antibodies, contributing to tissue damage in the small intestine (left); C: Cross-sectional structure of the large intestine, highlighting key layers: Mucosa, submucosa, muscle layer, and serosa, which play roles in barrier function and immune signaling (middle panel). Under normal conditions, a balanced immune environment in the intestinal mucosa is maintained by regulatory immune cells and intact epithelial and mucus barriers (rightmost panel). In inflammatory bowel disease, disruption of the mucus layer and epithelial barrier allows bacterial translocation, triggering immune dysregulation, recruitment of effector immune cells, and release of proinflammatory cytokines, leading to chronic inflammation.
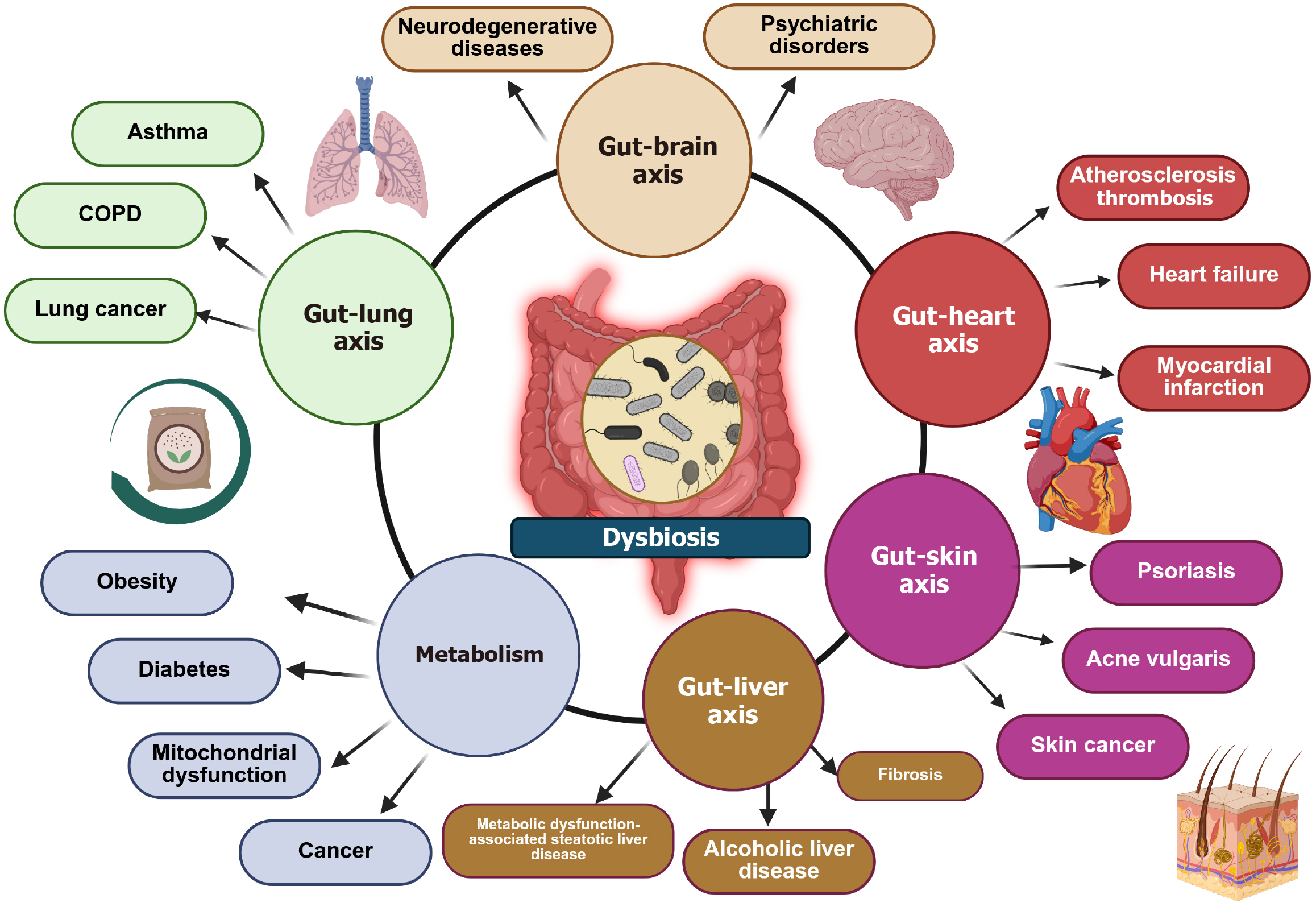
Figure 4 Depiction of the effects of dysbiosis on the human body.
This diagram illustrates the interconnected relationship between gut microbiota and various organ systems through distinct gut-organ axes. Central to this interaction is dysbiosis, an imbalance in gut microbiota, which contributes to multiple diseases. It is evident that changes of the microbiota have a systemic impact beyond the digestive system, demonstrating its role in health and disease through multiple organ interactions. Microbial metabolites act as an inflammatory stimulus which elicits a generalized inflammation. Furthermore, these metabolites (lipopolysaccharides, fatty acids, bile acids etc.) have to be digested finally arrive to the liver. This elicits further inflammation via activation of Kupffer cells.
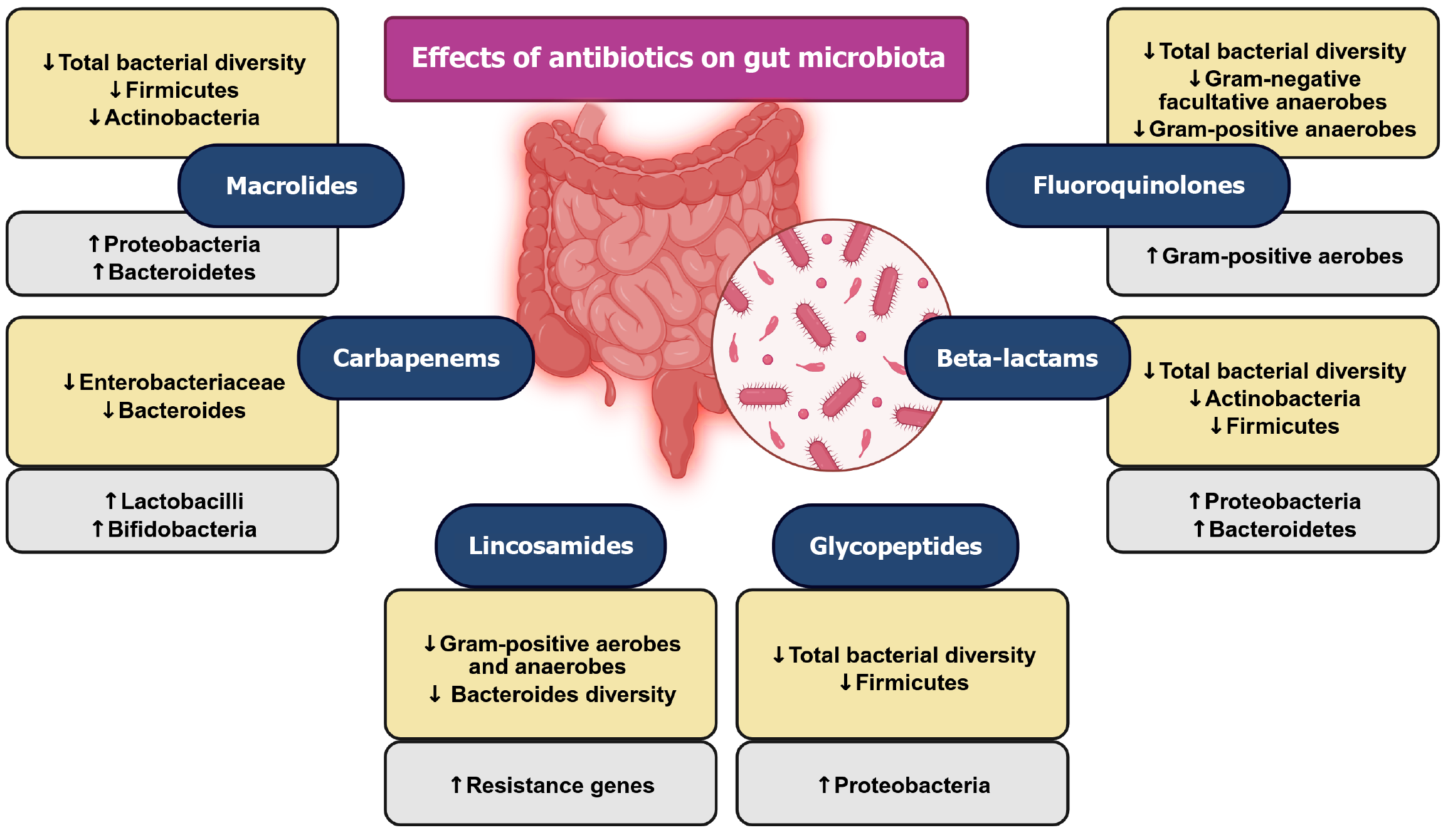
Figure 5 Schematic representations of the effects of antibiotics on gut microbiota.
This figure highlights the profound effects of antibiotics on gut microbiota composition and diversity. It emphasizes that antibiotic use disrupts the gut microbiome, potentially leading to long-term health consequences such as inflammation, metabolic disorders, and antibiotic resistance. In detail, most antibiotics reduce total bacterial diversity, often leading to microbial imbalances (dysbiosis). Several antibiotic classes, including macrolides, beta-lactams, and glycopeptides, promote an increase in Proteobacteria, which is associated with gut inflammation and dysbiosis. Many antibiotics, such as macrolides, beta-lactams, and glycopeptides, significantly reduce Firmicutes and Actinobacteria, which play a crucial role in gut homeostasis. While some antibiotics, like carbapenems and lincosamides, reduce Bacteroidetes diversity, others like macrolides promote their growth. While carbapenems decrease Enterobacteriaceae, they increase Lactobacilli, showing a mixed impact on gut flora.
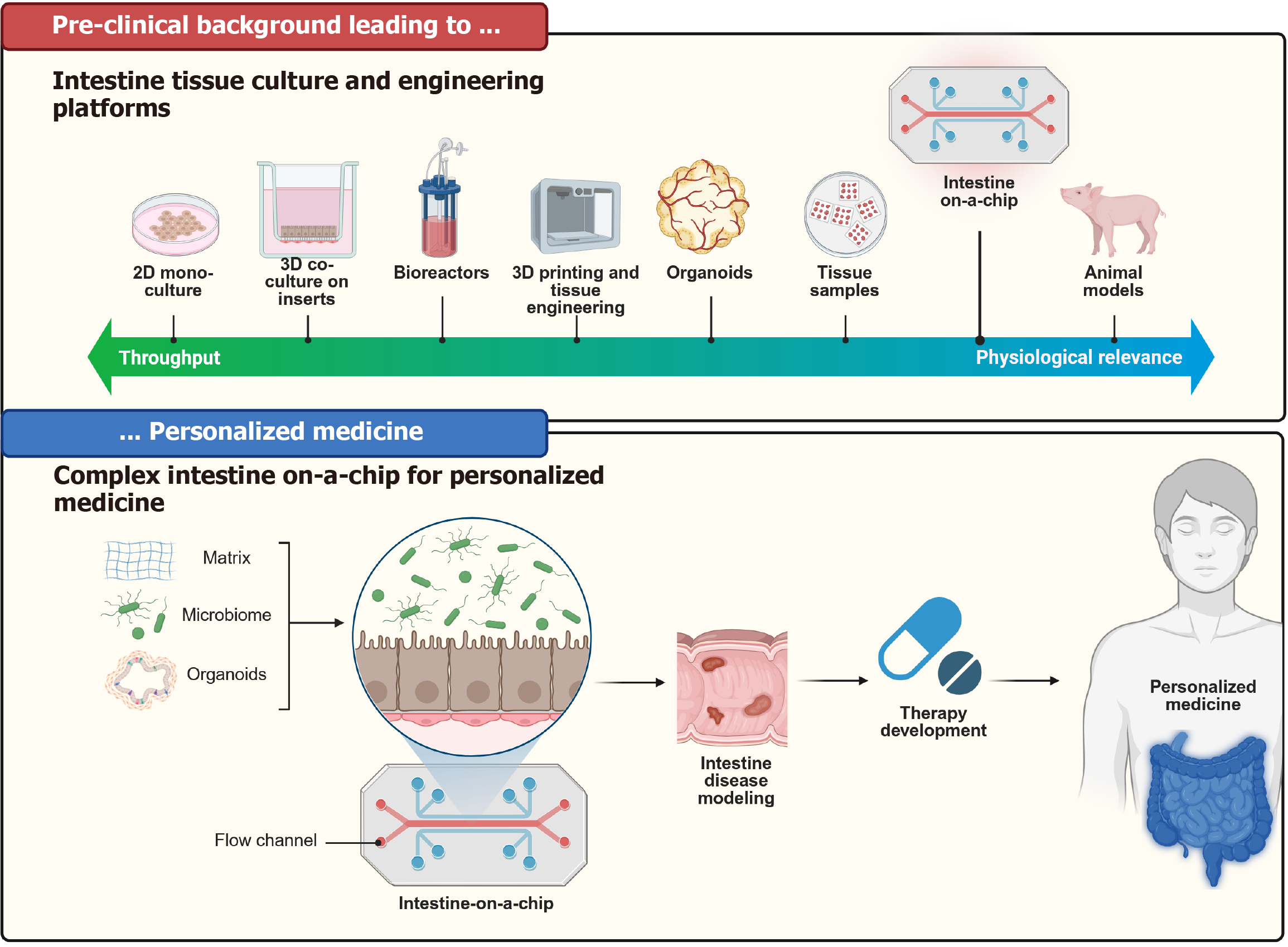
Figure 6 Representation of the milestones and different techniques of tissue culturing through time.
This diagram illustrates the evolution of intestine tissue culture and engineering platforms leading to the development of intestine-on-a-chip technology for personalized medicine. Pre-clinical background (top panel) showcases a progression from two-dimensional monocultures, three-dimensional co-cultures, bioreactors, three-dimensional printing, organoids, and tissue samples to intestine-on-a-chip and animal models, demonstrating a shift from high-throughput methods to increased physiological relevance. The bottom panel shows the intestine-on-a-chip, which integrates the microbiome, organoids, and extracellular matrix within a controlled flow channel, enabling intestine disease modelling. This facilitates therapy development, ultimately leading to personalized treatments tailored to individual patients. 2D: Two-dimensional; 3D: Three-dimensional.
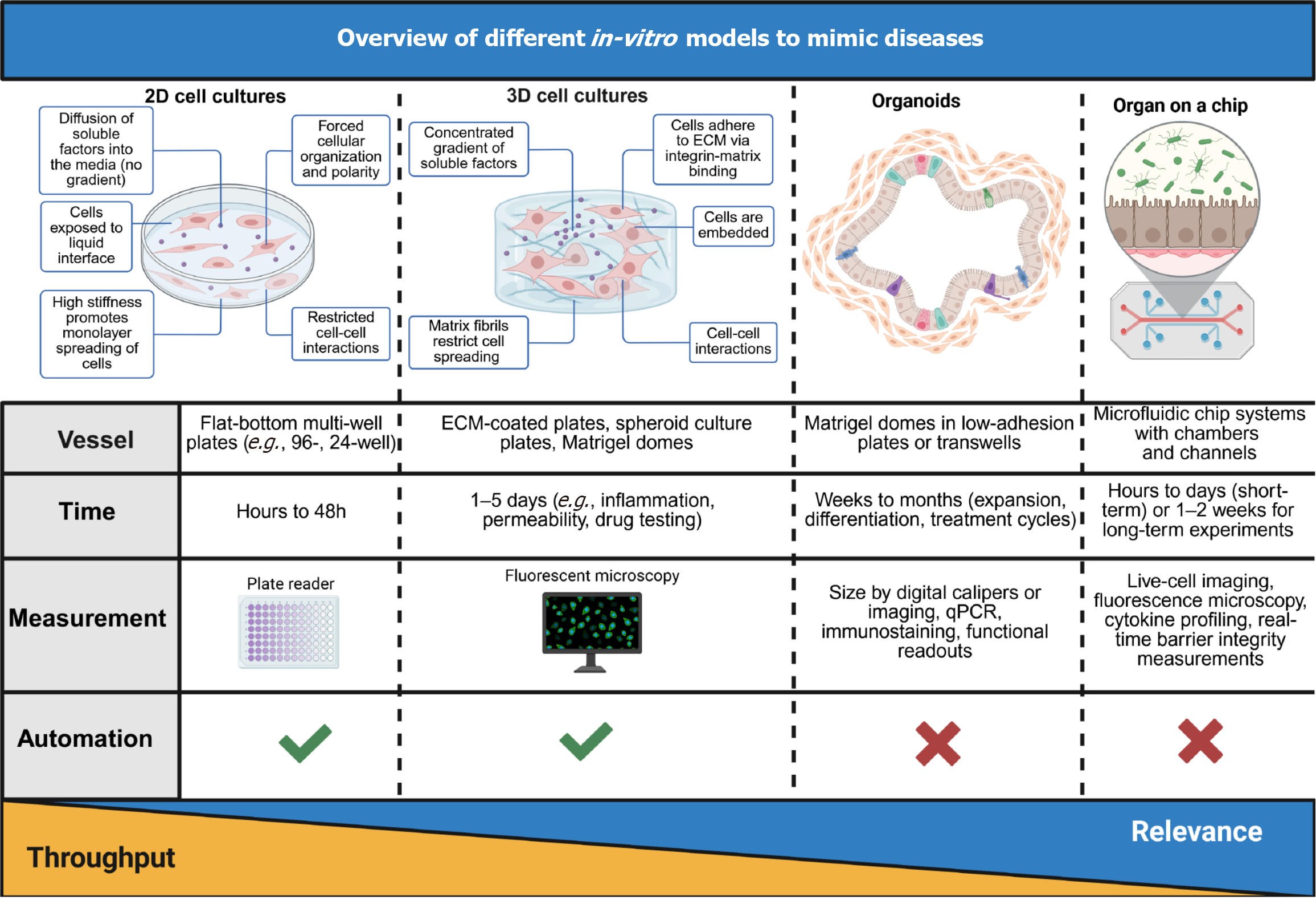
Figure 7 Overview of different in-vitro models to mimic diseases.
Four widely used in-vitro platforms (two-dimensional cell cultures, three-dimensional cell cultures, organoids, and organ-on-a-chip systems) are being compared based on their structural characteristics, vessel types, experimental duration, measurement techniques, and automation potential. The key features of each system are presented and the gradient bar at the bottom indicates the trade-off between throughput (highest in two-dimensional cultures) and physiological relevance (highest in organ-on-a-chip models). These in-vitro platforms vary in their ability to replicate tissue-specific microenvironments and are selected based on the desired biological complexity, readout needs, and disease modeling objectives. 2D: Two-dimensional; 3D: Three-dimensional.
- Citation: Skok K, Vihar B, Maver U, Gradišnik L, Bräutigam K, Trapecar M, Skok P. Gastrointestinal tract, its pathophysiology and in-vitro models: A “quick” reference guide to translational studies. World J Gastroenterol 2025; 31(28): 108297
- URL: https://www.wjgnet.com/1007-9327/full/v31/i28/108297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i28.108297