©The Author(s) 2024.
World J Gastroenterol. Apr 7, 2024; 30(13): 1815-1835
Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1815
Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1815
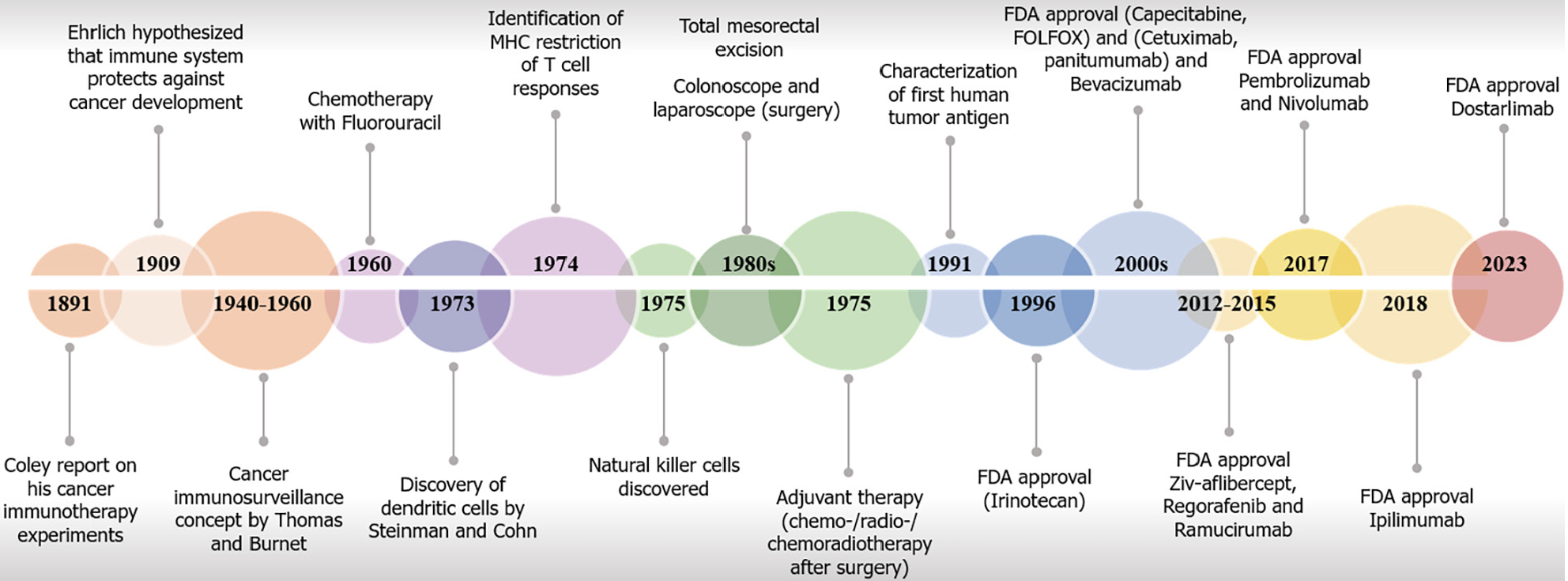
Figure 1 Timeline with key milestones in immuno-oncology research and United States Food and Drug Administration–approved immune checkpoint inhibitors in colorectal cancer.
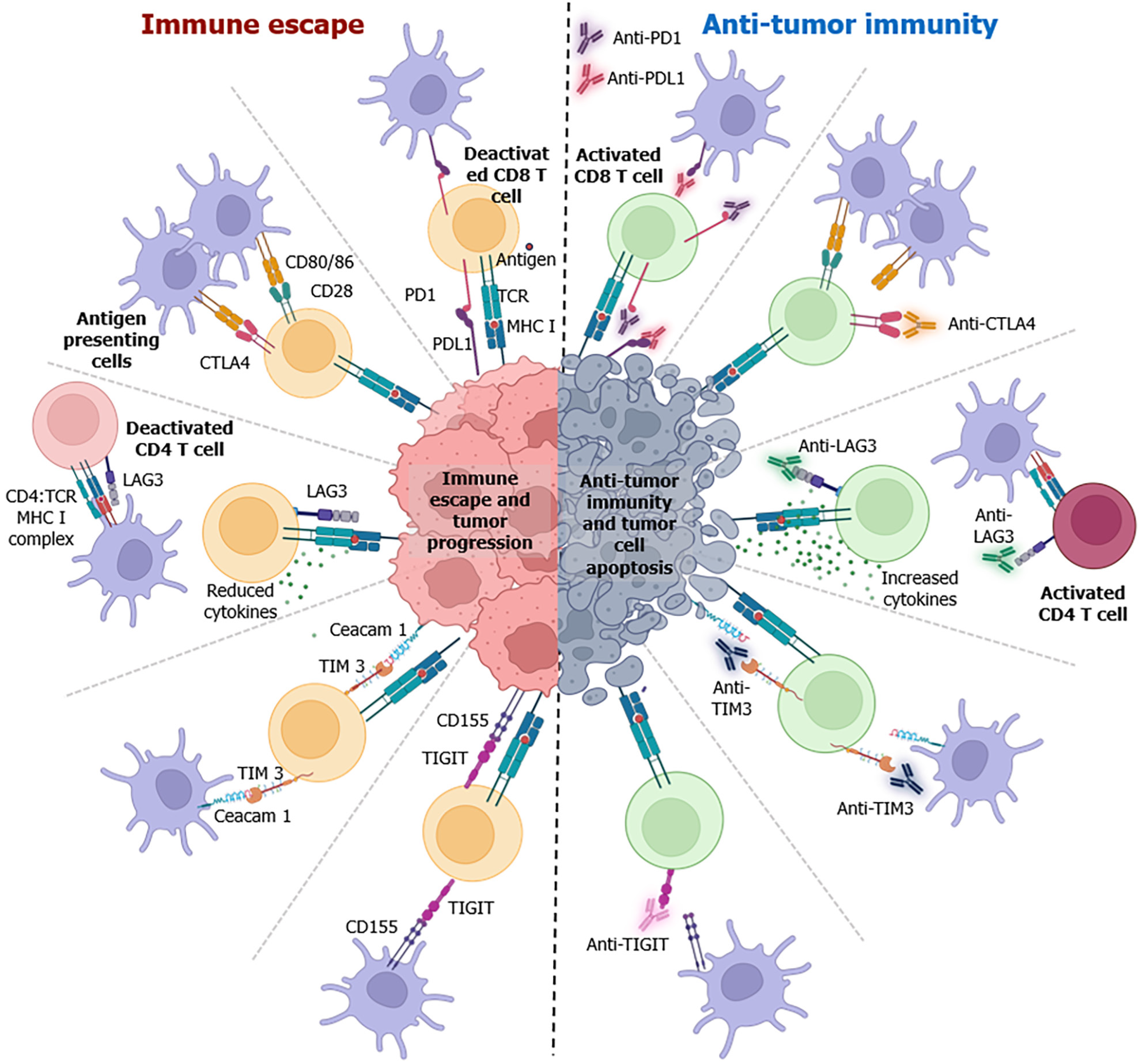
Figure 2 This figure presents a schematic diagram of the intricate interplay between immune checkpoints, immune cells, and malignant cells.
It also elucidates the underlying molecular mechanisms employed in immune-checkpoint blockade. In the tumor microenvironment, the interaction of immune checkpoints leads to immune suppression and facilitates tumor progression (depicted on the left side of the diagram). Conversely, the administration of immune checkpoint inhibitions reverses the immune escape mechanism, fostering increased anti-tumor immunity and triggering tumor apoptosis (depicted on the right side of the diagram). FDA: Food and Drug Administration.
- Citation: Sharma S, Singh N, Turk AA, Wan I, Guttikonda A, Dong JL, Zhang X, Opyrchal M. Molecular insights into clinical trials for immune checkpoint inhibitors in colorectal cancer: Unravelling challenges and future directions. World J Gastroenterol 2024; 30(13): 1815-1835
- URL: https://www.wjgnet.com/1007-9327/full/v30/i13/1815.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i13.1815