©The Author(s) 2024.
World J Gastroenterol. Apr 7, 2024; 30(13): 1780-1790
Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1780
Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1780
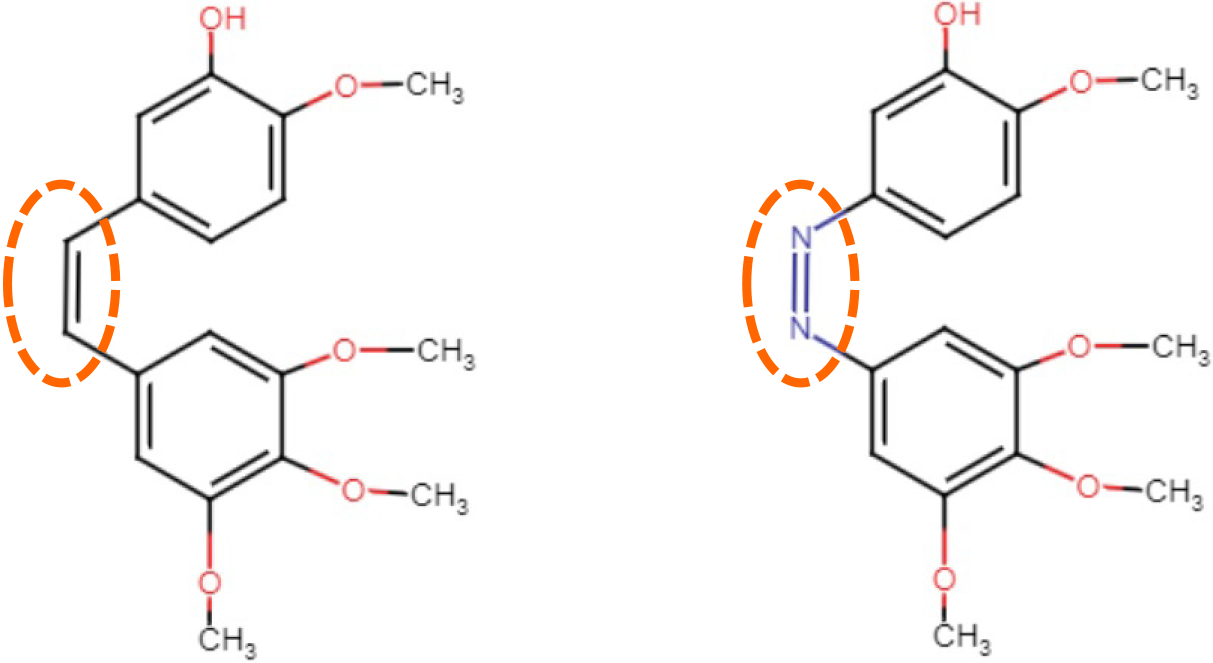
Figure 1 The chemical structures of combretastatin CA-4 (left) and photostatin photoswitchable photostatins-1[56].
Both compounds have either the C=C or N=N double-bond that can induce photo-isomerization by light (circle).
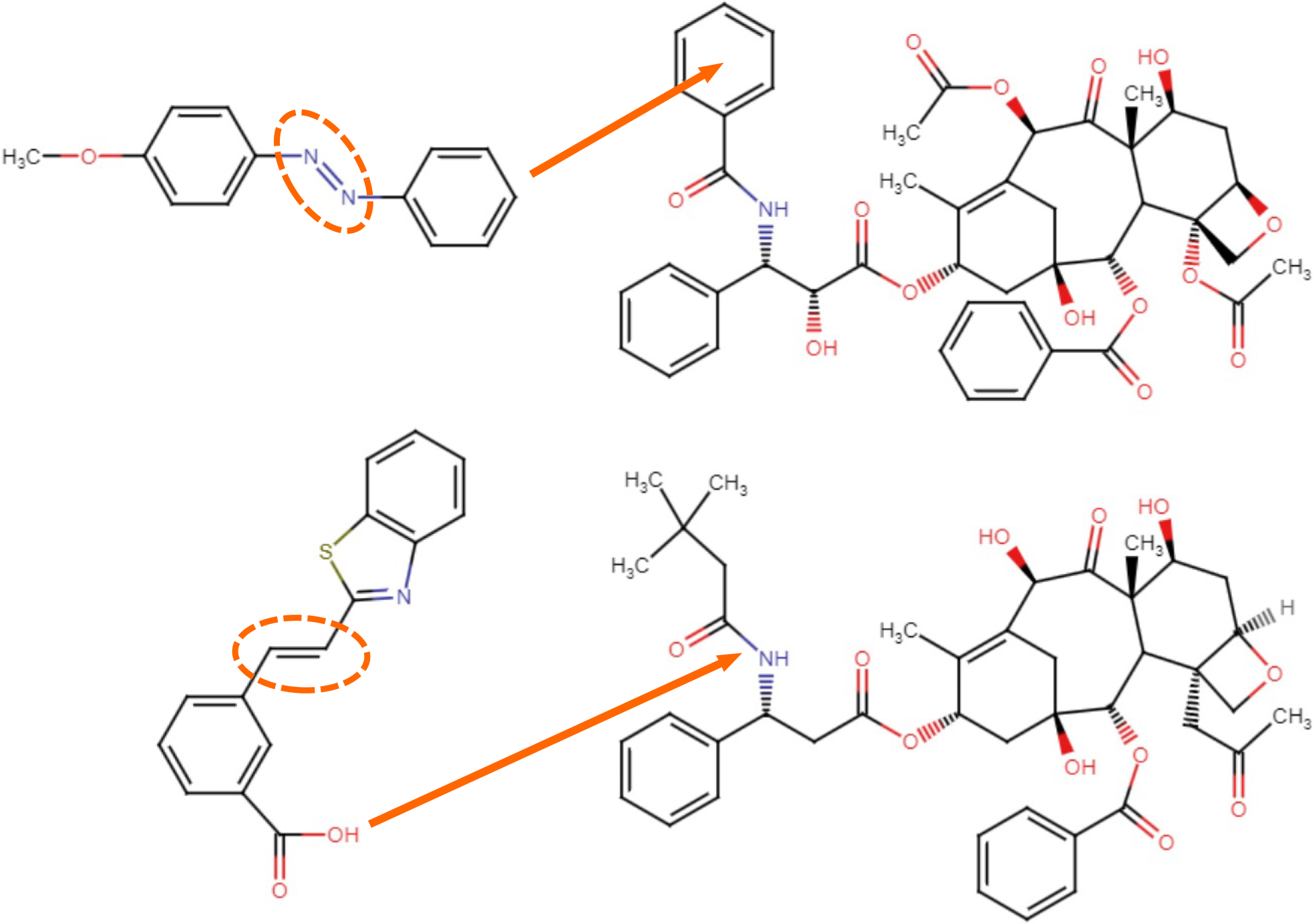
Figure 2 The chemical structures of paclitaxel (top right) with an azobenzene derivative (top left) and docetaxel with a styrylbenzothiazole derivative (bottom).
The photo-switchable C=C or N=N double-bond is highlighted (dotted circle). Each group is conjugated to the left arm of taxanes (arrows), resulting AzTax3MP[58] and SBTax[60], respectively.
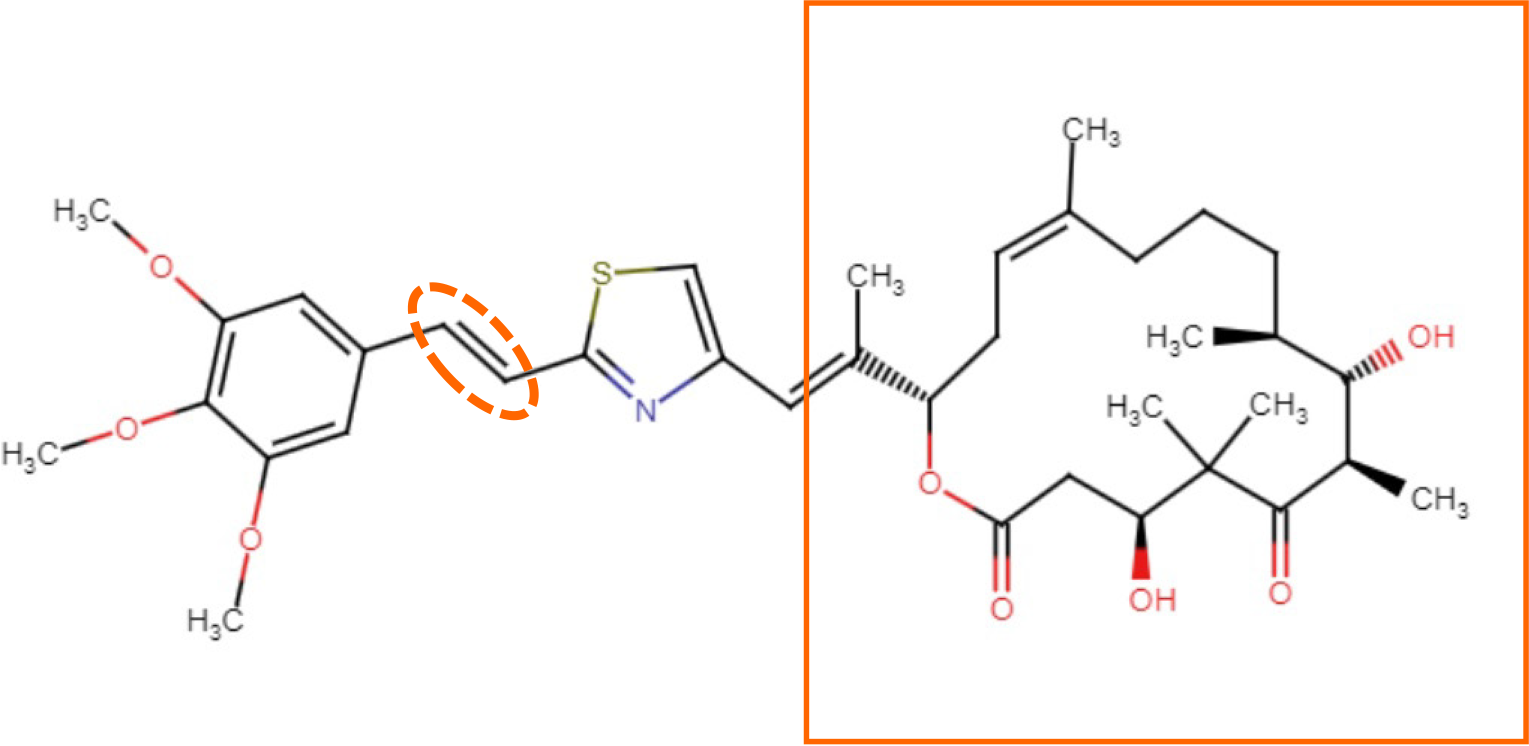
Figure 3 The chemical structures of STEpo2[60], a photo-switchable derivative of epothilone D.
The photo-switchable C=C double-bond (dotted circle) in the attached styrylthiazole group. The rectangle indicates the core structure of epothilone D.
- Citation: Kita K, Burdowski A. Recent clinical trials and optical control as a potential strategy to develop microtubule-targeting drugs in colorectal cancer management. World J Gastroenterol 2024; 30(13): 1780-1790
- URL: https://www.wjgnet.com/1007-9327/full/v30/i13/1780.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i13.1780