©The Author(s) 2023.
World J Gastroenterol. Aug 28, 2023; 29(32): 4831-4850
Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4831
Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4831
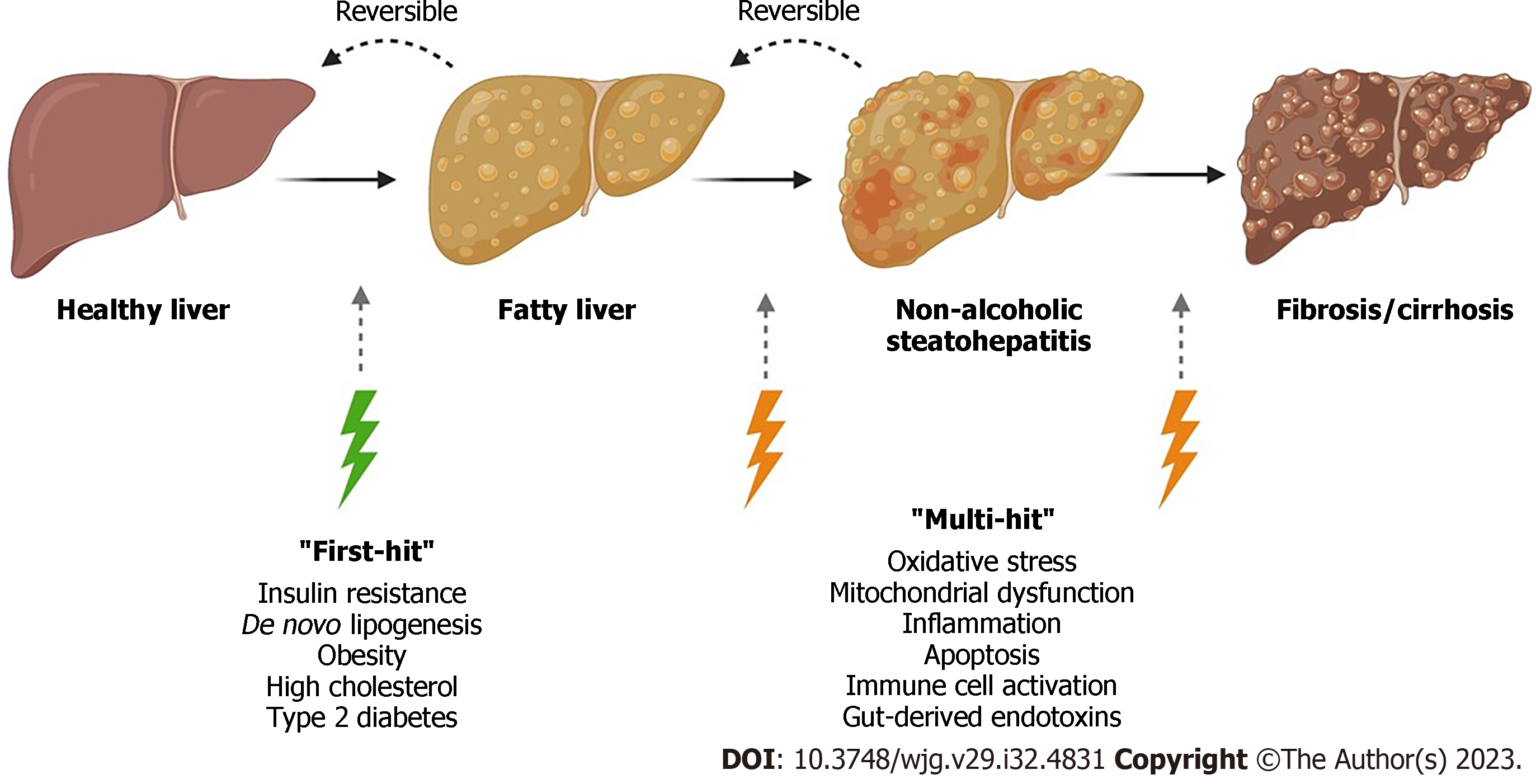
Figure 1 Overview of the current understanding of non-alcoholic fatty liver disease progression.
Non-alcoholic fatty liver disease (NAFLD) encompasses a range of liver damage, from simple accumulation of fat in liver cells, named steatosis, to more severe forms of the diseases such as steatohepatitis, involving inflammation which can lead to fibrosis and cirrhosis. In the “multiple-hit” theory of progression, the first cause or “first-hit” in NAFLD is insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. As the first hit occurs, free fatty acids are stored in the liver as triglycerides, resulting in simple steatosis. Disease progresses when multiple factors, or “multi-hits”, such as oxidative stress, inflammatory mediators, apoptosis, and mitochondrial dysfunction cause liver damage (created with BioRender.com).
Figure 2 Overview of mitochondrial dynamics in non-alcoholic fatty liver disease.
The accumulation of triglycerides and free fatty acids (FFAs) in the liver are converted to fatty acyl-CoA, which is then transported to the mitochondria for β-oxidation, generating acetyl-CoA. However, increased accumulation of FFA can cause insufficient hepatic β-oxidation, triggering inflammation and oxidative stress. Acetyl-CoA enters the mitochondrial tricarboxylic acid cycle allowing continuation of gluconeogenesis. The by-product nicotinamide adenine dinucleotide, produced though β-oxidation are transported to the electron transport chain (ETC) for oxidative phosphorylation. Disruption and dysfunction of the ETC can cause electron leakage and hepatocyte damage leading to reactive oxygen species production and oxidative stress. Accumulation of FFAs can cause mitochondrial damage, resulting in increased mitochondrial fission and degradation via mitophagy processes (created with BioRender.com). ROS: Reactive oxygen species; NADH: Nicotinamide adenine dinucleotide; TCA: Tricarboxylic acid; ADP: Adenosine diphosphate; ATP: Adenosine triphosphate.
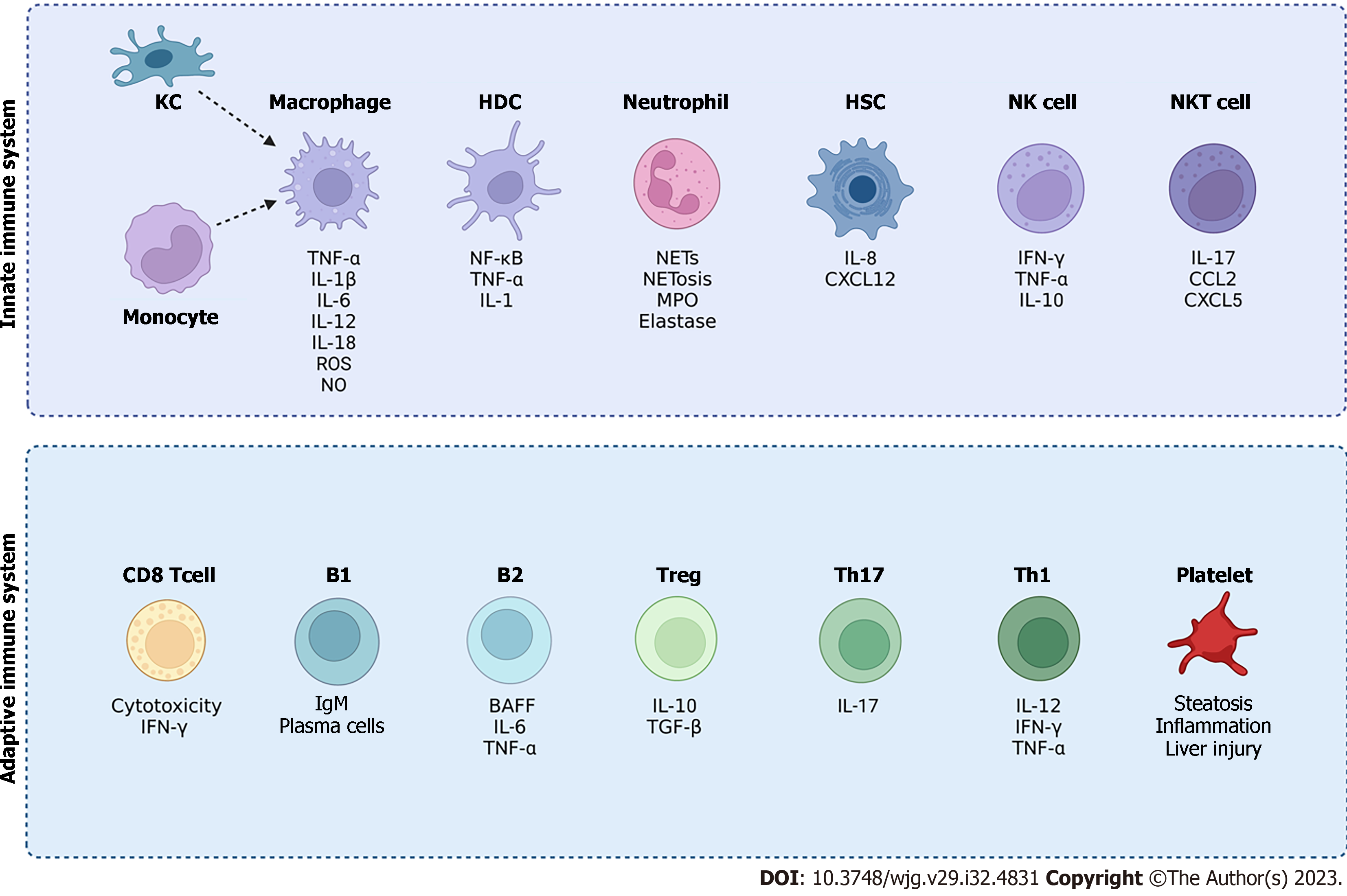
Figure 3 Overview of innate and adaptive immune cells involved in non-alcoholic fatty liver disease (NAFLD).
Activation of immune cells can promote inflammation and liver injury in NAFLD. The innate immune system cells include Kupffer cells, monocytes, and macrophages as well as hepatic dendritic cells, neutrophils and natural killer cells. Natural killer T cells provide a bridge between both the innate and adaptive immune system. The adaptive immune system includes T cells such as CD8 T cells and CD4 T cells (Th1, Th17 and regulatory T cells). The adaptive immune system also comprises of B1 and B2 cells as well as platelets. Although the contribution of immune cells to the development of NAFLD has been explored, the crosstalk between the distinctive immune cell subsets in the pathogenesis of NAFLD requires further investigation (created with BioRender.com). KC: Kupffer cells; TNF-α: Tumour necrosis factor-alpha; IFN-γ: Interferon-γ; IL: Interleukin; ROS: Reactive oxygen species; NO: Nitric oxide; NETs: Neutrophil extracellular traps; NF-κB: Nuclear factor-kappa B; HDC: Hepatic dendritic cells; MPO: Myeloperoxidase; HSC: Hepatic stellate cells ; CXCL: C-X-C motif chemokine ligand; BAFF: B cell activating factor; TGF-β: Transforming growth factor-β; NK: Natural killer; NKT: Natural killer T cells; Treg: Regulatory T cells.
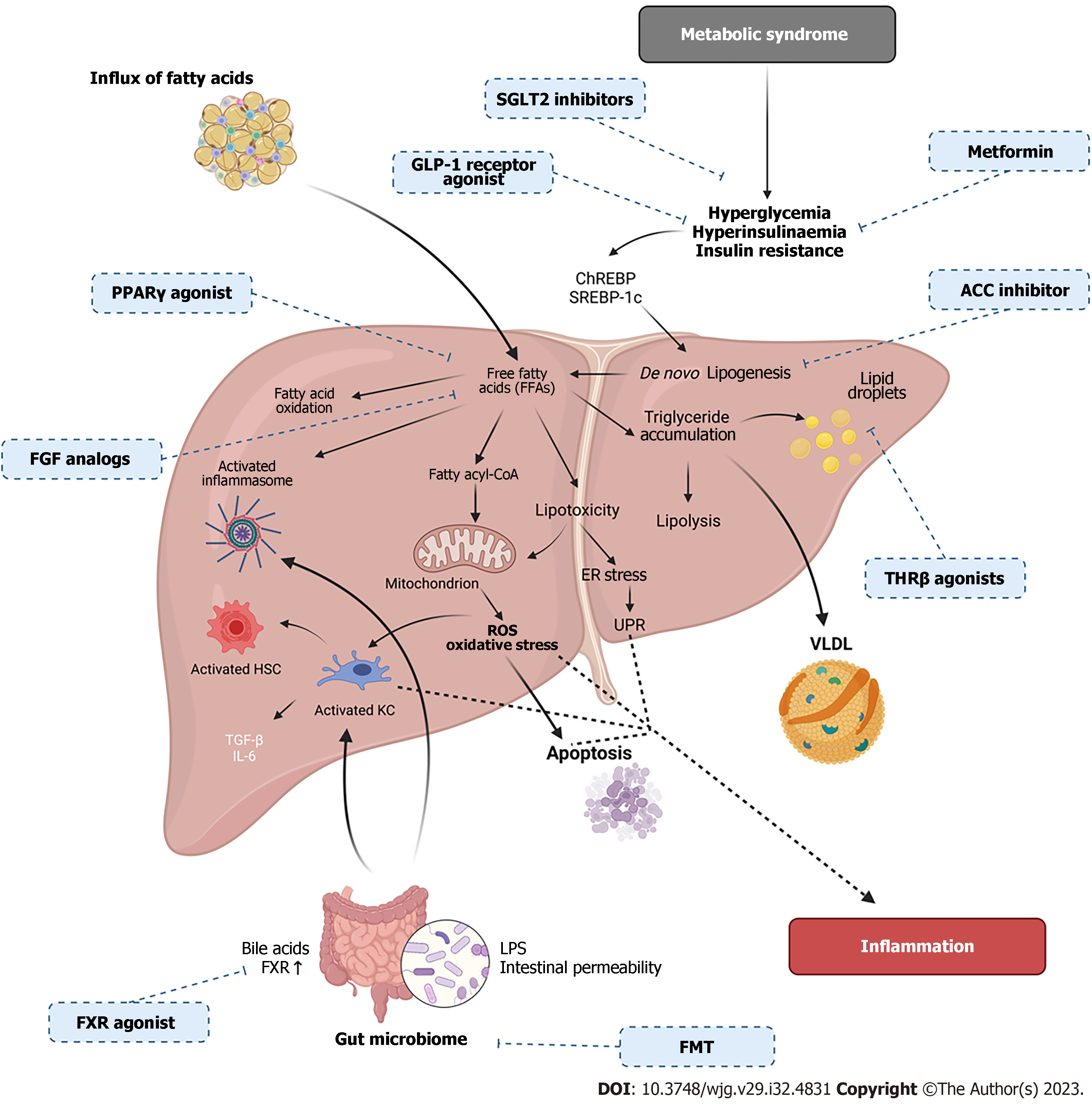
Figure 4 Overview of the metabolic and molecular mechanisms occurring in non-alcoholic fatty liver disease pathogenesis with therapeutic targets.
Non-alcoholic fatty liver disease (NAFLD) is a multifactorial disease, including metabolic syndrome, insulin resistance (IR) and gut microbiome changes. IR and metabolic syndrome cause increased free fatty acids (FFAs) which lead to endoplasmic reticulum stress, reactive oxygen species accumulation, oxidative stress, apoptosis, immune cell activation and inflammasome activation. Changes in the gut barrier can cause changes to bile acids and intestinal permeability causing lipopolysaccharides translocation from the gut causing immune activation, thus, inducing inflammation. FFA’s can also activate transcription factors such as sterol regulatory binding protein-1c and carbohydrate response element binding protein causing the storage of FFAs as triglycerides in lipid droplets. Accumulated triglycerides can be exported as very low-density lipoprotein or oxidized by mitochondrial β-oxidation. Pharmacologic treatments for NAFLD are identified with corresponding drug mechanism of action (created with BioRender.com). ROS: Reactive oxygen species; LPS: Lipopolysaccharide; UPR: Unfolded protein response; FMT: Faecal microbiota transplant; VLDL: Very low-density lipoproteins; PPAR-γ: Peroxisome proliferator-activated receptor-γ; KC: Kupffer cells; HSC: Hepatic stellate cells; FXR: Farnesoid X Receptor; SGLT2: Sodium-glucose cotransporter 2; SREBP-1c: Sterol regulatory binding protein-1c; ChREBP: Carbohydrate response element binding protein.
- Citation: Petagine L, Zariwala MG, Patel VB. Non-alcoholic fatty liver disease: Immunological mechanisms and current treatments. World J Gastroenterol 2023; 29(32): 4831-4850
- URL: https://www.wjgnet.com/1007-9327/full/v29/i32/4831.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i32.4831