©The Author(s) 2022.
World J Gastroenterol. Mar 7, 2022; 28(9): 897-908
Published online Mar 7, 2022. doi: 10.3748/wjg.v28.i9.897
Published online Mar 7, 2022. doi: 10.3748/wjg.v28.i9.897
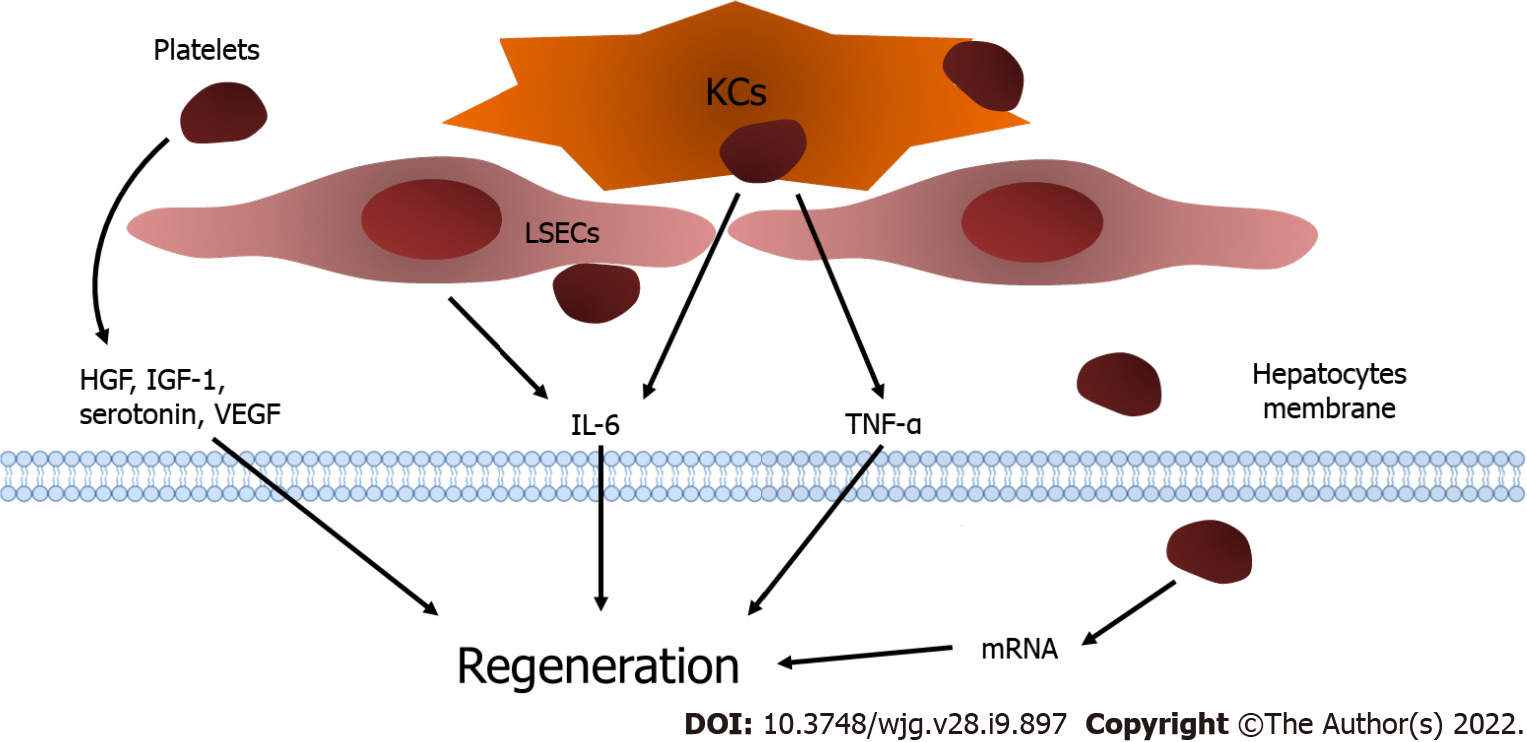
Figure 1 Platelets and liver regeneration.
Platelets translocate into the space of Disse and release insulin-like growth factor-1, hepatocyte growth factor, and vascular endothelial growth factor. The direct contact of platelets with liver sinusoidal endothelial cells (LSECs) results in the excretion of interleukin-6 (IL-6) from LSECs. In addition, the attachment of platelets activates Kupffer cells (KCs) and enhances the release of tumor necrosis factor-alpha and IL-6 from KCs to promote liver regeneration. Moreover, platelets are internalized into hepatocytes and trigger the functional transfer of messenger RNA stored in platelets, which stimulates hepatocyte proliferation. KCs: Kupffer cells; LSECs: Liver sinusoidal endothelial cells; IGF-1: Insulin-like growth factor-1; HGF: Hepatocyte growth factor; VEGF: Vascular endothelial growth factor; LSECs: Liver sinusoidal endothelial cells; IL-6: Interleukin-6; KCs: Kupffer cells; TNF-α: Tumor necrosis factor-alpha.
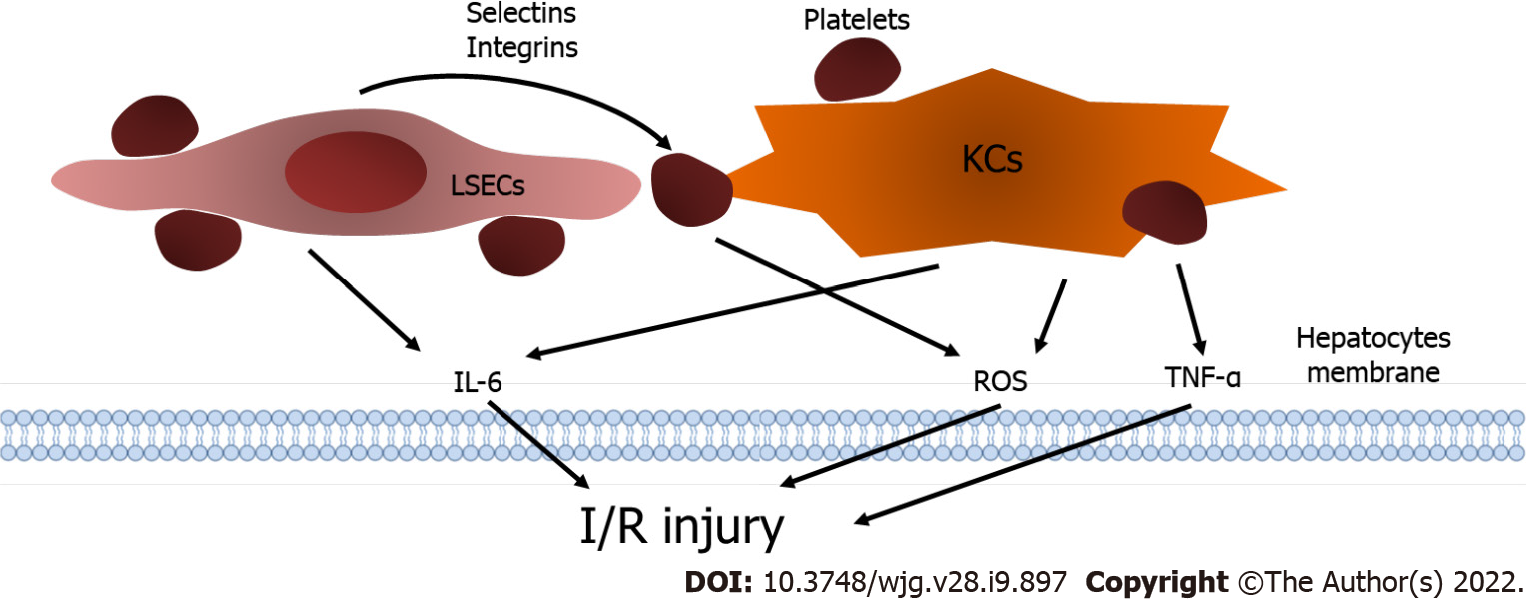
Figure 2 Platelets and ischemia/reperfusion injury.
Liver sinusoidal endothelial cells (LSECs) express selectins and integrins to stimulate the interaction between platelets and LSECs, and platelets result in the excretion of interleukin-6 (IL-6) from LSECs. The generation of tumor necrosis factor-alpha, IL-6 and reactive oxygen species (ROS) from KCs is elevated after the cooperative effect between platelets and KCs. Furthermore, platelets can produce ROS independently and consequently aggravate ischemia/reperfusion injury. KCs: Kupffer cells; LSECs: Liver sinusoidal endothelial cells; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-alpha; ROS: Reactive oxygen species; I/R injury: Ischemia/reperfusion injury.
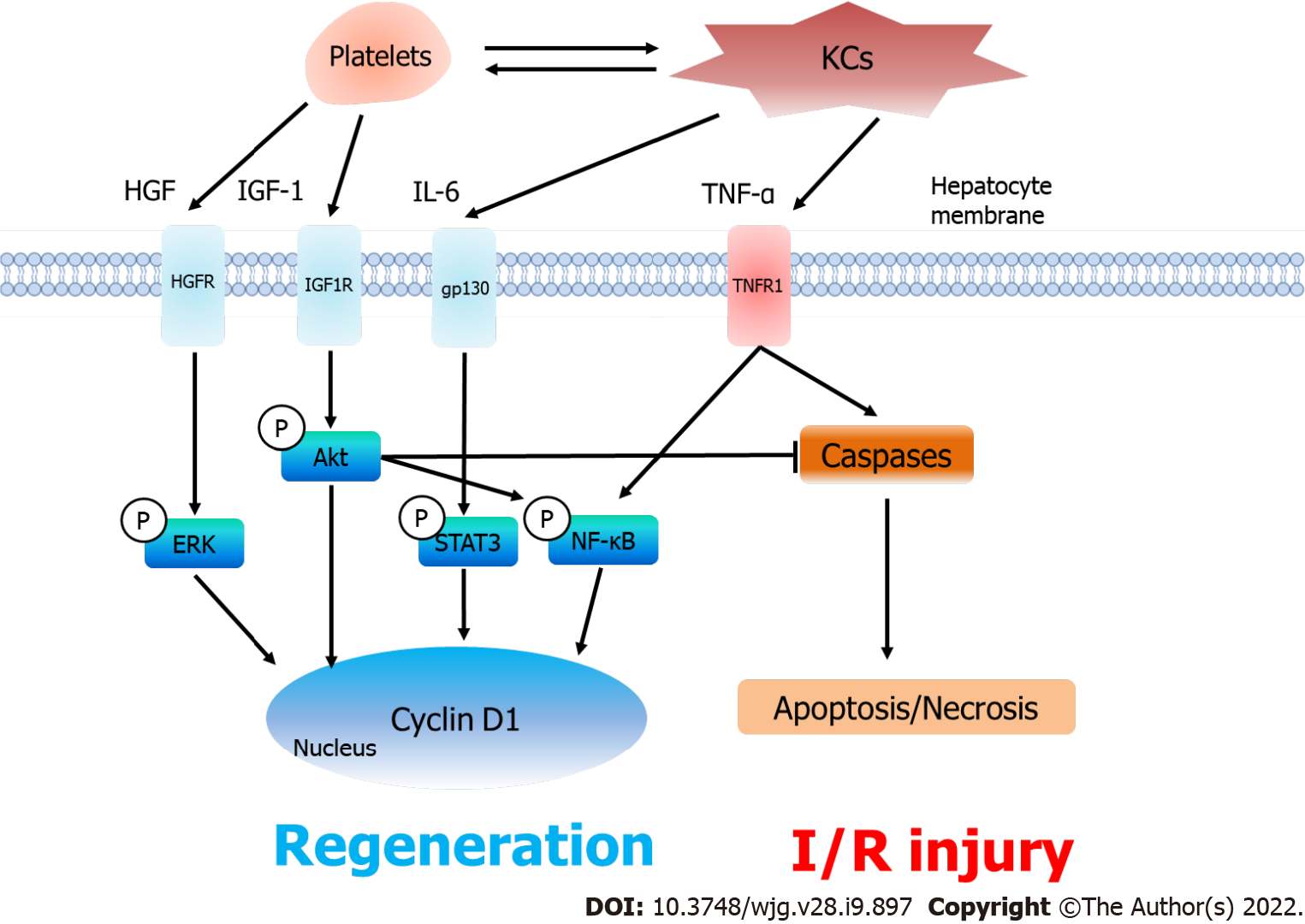
Figure 3 Platelets, liver regeneration and ischemia/reperfusion injury after partial liver transplantation.
After accumulating in the liver graft, platelets excrete hepatocyte growth factor (HGF) and insulin-like growth factor-1 (IGF-1) and collaborate with KCs to increase the release of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). As a result, the serum levels of HGF, IGF-1, IL-6 and TNF-α increase under thrombocytosis, which consequently induces the phosphorylation of the ERK, Akt, STAT3 and nuclear factor-kappa B signaling pathways to promote liver regeneration (Cyclin D1). On the other hand, platelets do not aggravate in ischemia/reperfusion injury. The phosphorylated Akt pathway inhibits TNF-α-induced apoptosis and necrosis in the liver graft. KCs: Kupffer cells; HGF: Hepatocyte growth factor; IL-6: Interleukin-6; IGF-1: Insulin-like growth factor-1; TNF-α: Tumor necrosis factor-alpha; HGFR: HGF receptor; IGF1R: IGF-1 receptor; gp130: Glycoprotein 130; TNFR1: Tumor necrosis factor receptor; ERK: Extracellular signal-regulated kinase; STAT3: Signal transducer and activator of transcription 3; NF-κB: Nuclear factor-kappa B; Caspase: Cysteinyl aspartate specific proteinase; I/R injury: Ischemia/reperfusion injury.
- Citation: Liang C, Takahashi K, Furuya K, Ohkohchi N, Oda T. Dualistic role of platelets in living donor liver transplantation: Are they harmful? World J Gastroenterol 2022; 28(9): 897-908
- URL: https://www.wjgnet.com/1007-9327/full/v28/i9/897.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i9.897