©The Author(s) 2022.
World J Gastroenterol. Aug 7, 2022; 28(29): 3994-4006
Published online Aug 7, 2022. doi: 10.3748/wjg.v28.i29.3994
Published online Aug 7, 2022. doi: 10.3748/wjg.v28.i29.3994
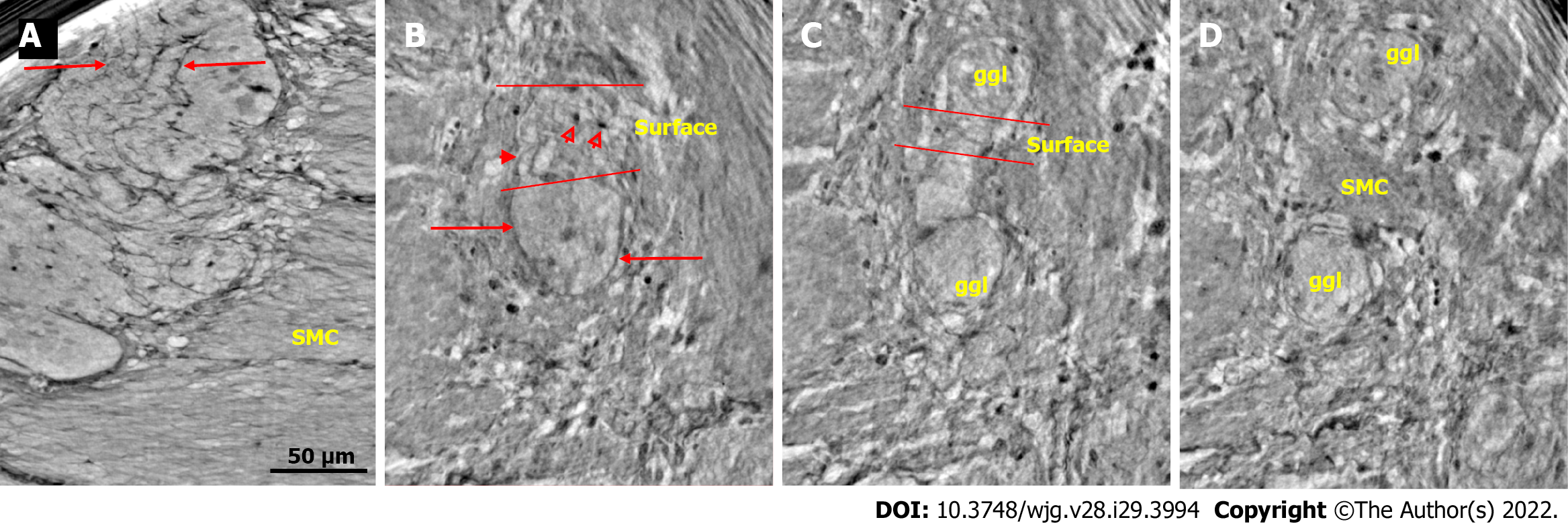
Figure 1 Ganglion from healthy human ileum.
A: Part of the myenteric ganglion in two portions. Quite regular, almost parallel, thin cytoplasmic projections of telocytes (telopodes) with short, thin ”spines” on the surface are observed (between arrows). The scale bar (50 μm) was applied to all subfigures; B: A small portion of the ganglion containing two neurons. On the surface of the ganglion (between the two lines), the cellular nuclei of the two telocytes were observed as dark spots (empty arrowheads). Telopodes radiate from their body. Arrows indicate the telopodes around the ganglion. A telopode can be followed toward the surface (arrowhead); C: 16.9 μm deeper from Figure B, two portions appeared with thin telopodes on the surface; D: 10.1 μm deeper from Figure C, SMCs are observed between the two portions. B-D are representative virtual slices from a series covering 27.0 μm thickness of the ganglion. Ggl: Ganglion; SMC: Smooth muscle cells.
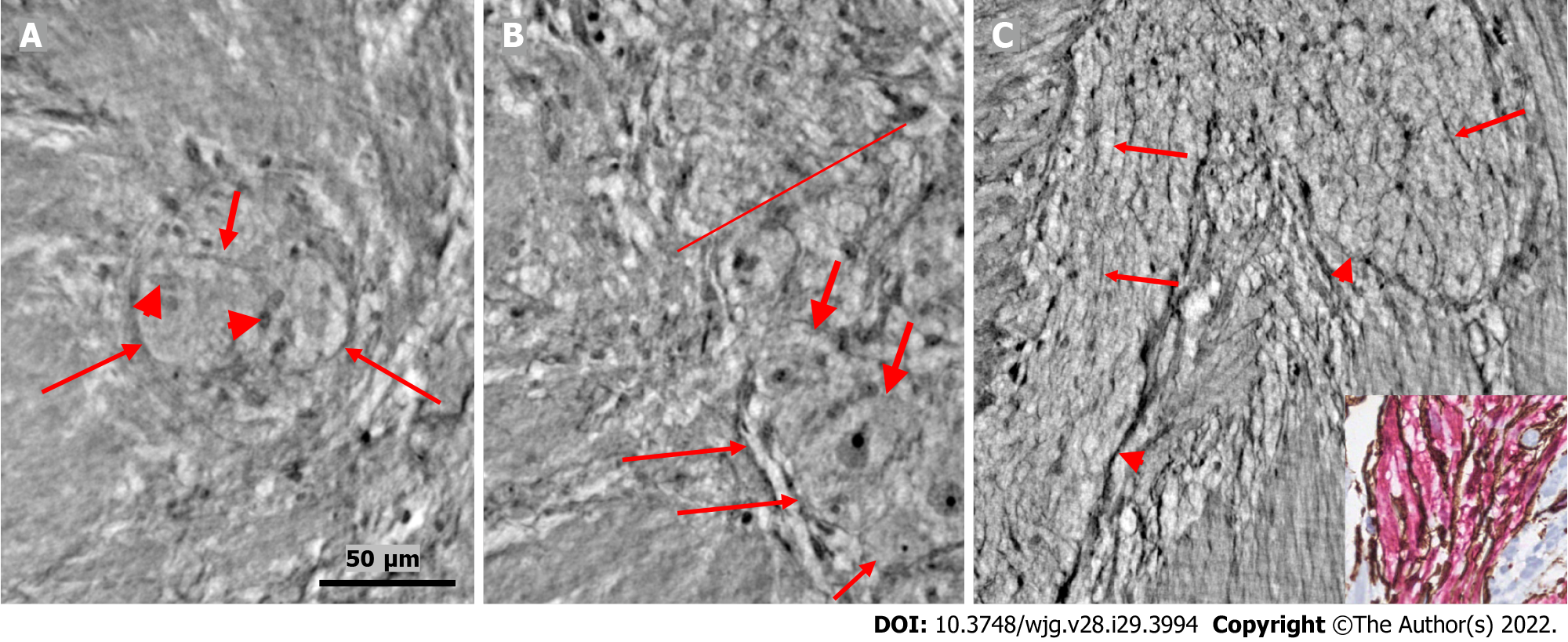
Figure 2 Ganglion and fascicle from healthy human ileum.
A: Within a portion of the ganglion, there is a neuron with an axon (thick arrow). Arrowheads indicate glial cells. Thin arrows indicate a single layer of telopodes at the border of the ganglion. The scale bar (50 µm) was applied to all subfigures; B: Part of a ganglion with several large (thick arrows) and medium-large (short thin arrow) neurons. The nuclei contain dark nucleoli. The cytoplasm is slightly granulated. Above the straight line, a small area of the neuropil was observed. Long thin arrows indicate the double-cell layer at the border; C: Normal nerve fascicle and thicker nerve with telopodes separating axons (arrows). Note the single layer of telopodes around the fascicle and nerve (arrowheads). Inset: Normal nerve fascicles stained with double immunohistochemistry. Telocytes run parallel to Schwann cells (light microscopy; S100: Schwann cells red; CD34: Telocytes brown).
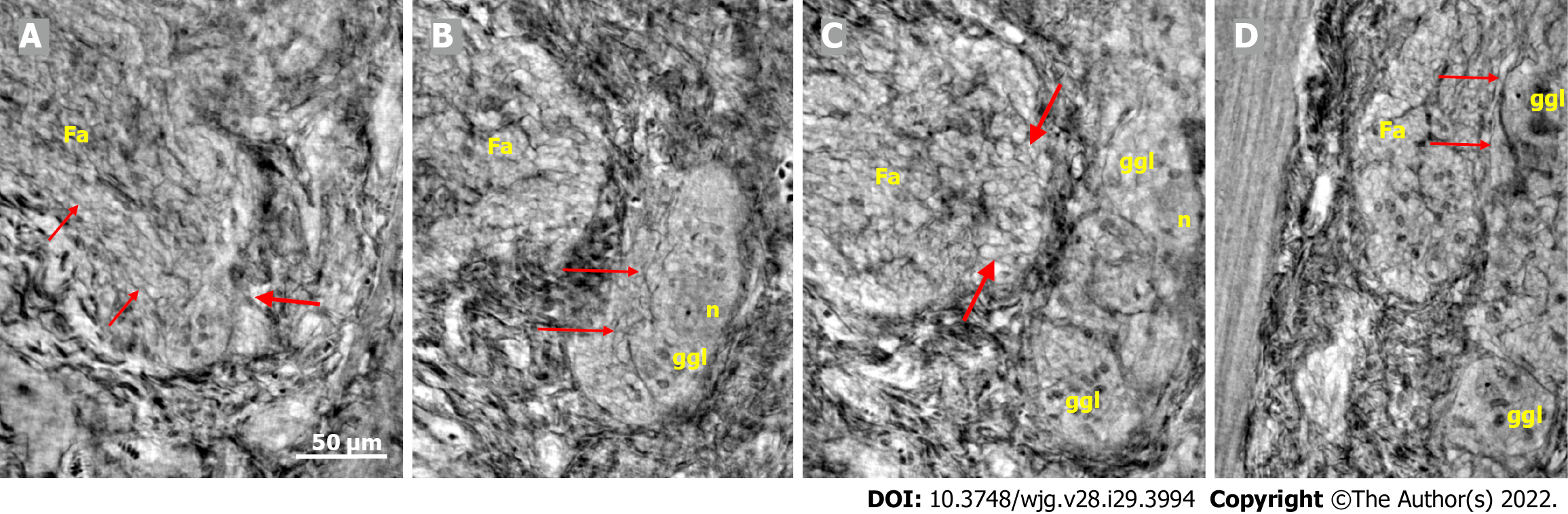
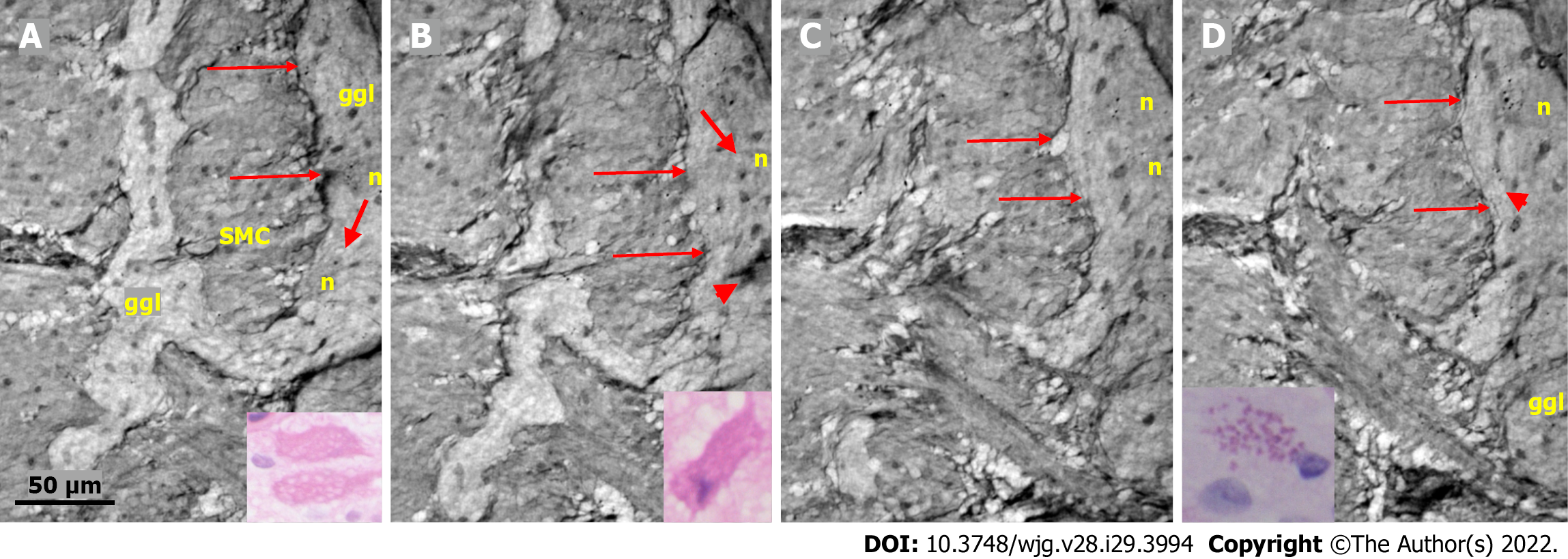
Figure 3 Series of virtual slices covering 113.
6 μm thickness at the border between the nerve fascicle and the ganglion. A: Thick fascicle with parallel telopodes (thin arrows). There is a small peripheral part of the ganglion (thick arrow); B: 62.2 μm deeper from Figure A. Connective tissue cells between the fascicle and the superficial part of the ganglion. Normal large neurons and nuclei of several glial cells in the ganglion. A network of telopodes is present on the surface of the ganglion (arrows); C: 11.5 μm deeper from Figure B. The thickness of the connective tissue is diminished between the fascicle and the periphery of the ganglion, with two neurons and small glial cells. Transversally cut vesicle-like axons (between the arrows) on the right end of the fascicle; D: 39.9 μm deeper from Figure C, the fascicle and ganglion are united. Thin arrows show telopodes. The scale bar (50 µm) applies to all the subfigures. Fa: Fascicle; ggl: Ganglion; n: Neuron.
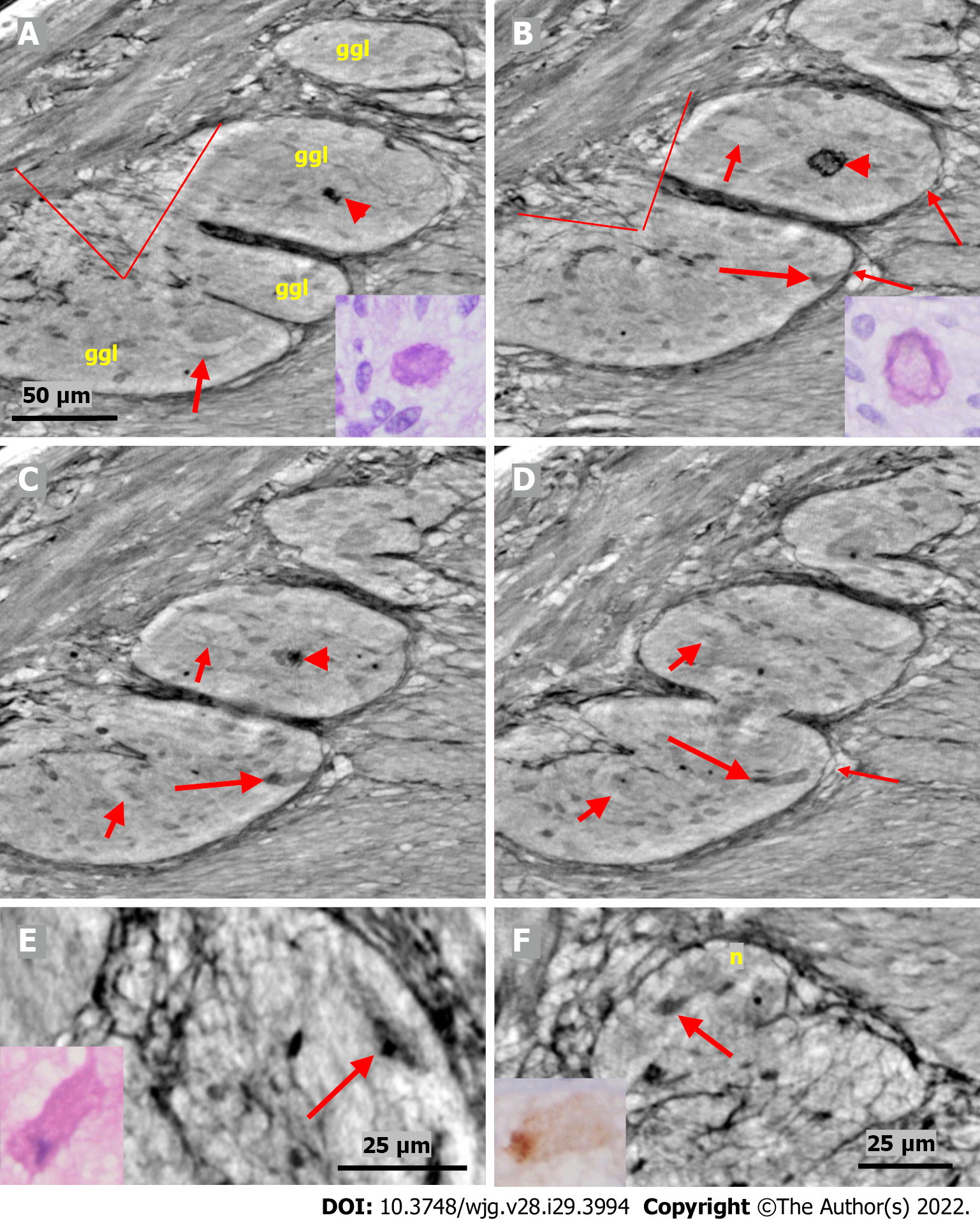
Figure 4 Series of virtual slices covering 17.
2 μm thickness of the ganglion from one patient. A: Four portions of the ganglion. There is a dark body at the center of one of these areas (arrowheads). An arrow indicates a neuron with a very pale homogeneous cytoplasm below the nucleus. Between the straight lines, a network of telopodes is observed on the ganglion surface. Incomplete septa between portions. Scale bar (50 µm) is applied to subfigures A-D. Inset: Hyaline body in the ganglion [1st level of serial sections; light microscopy; periodic acid-Schiff with diastase (PAS-D) staining]; B: 6.8 μm from Figure A., three portions of the ganglia are completely separated, and the surface area is smaller (between straight lines). The central dark body shows an irregular dense outer layer and central light area (arrowhead). A new neuron with pale cytoplasm is seen in the middle portion (short arrow). Intact telopodes form the border between the ganglion and the periganglional connective tissue rim (thin arrows). An almost triangle-shaped moderately dense area is seen below the telopode telopode corresponding to an apoptotic neuron (long arrow; see also C and D). Inset: The central region of the hyaline body is negative, whereas the outer layer is positive with PAS-D staining (2nd level of serial sections, light microscopy; PAS-D); C: 4.0 μm from Figure B. The hyaline body is without a central pale area (arrowhead), with a semi-dense irregular half-moon to the left. The two neurons had a pale cytoplasm (short arrows). A dark pyknotic nucleus appeared in the semi-dense cytoplasm at the border showing an apoptotic neuron corresponding to the “dark triangle” in Figure B (long arrow); D: 6.4 μm deeper from Figure C. Two of the portions are united. Nuclei are present in neurons with pale cytoplasm (short arrows). Intact telopodes are present between the degenerated apoptotic cells (long arrow) and the periganglional space (thin arrow); E: Digital magnification of a shrunken neuron with a darker cytoplasm, small vacuoles, and enlarged nucleolus. Scale bar: 25 µm Inset: Pre-apoptotic neurons with pyknotic nucleus and vacuole-containing amphophilic cytoplasm (light microscopy; H&E staining); F: There is a homogeneous circumscribed “inclusion” in the neuron, suggesting a sequestosome, as seen in the inset (arrow). Scale bar: 25 µm. Inset: Large p62+ aggregates in neurons (arrow; light microscopy; p62 immunohistochemistry). Scales bars of 50 or 25 µm included. Ggl: Ganglion; n: Neuron; PAS-D: Periodic acid-schiff with diastase.
Figure 5 Series of virtual slices covering 19.
9 μm thickness of the ganglion from one patient. A: Two portions of the ganglion with smooth muscle cells between them. Long arrows indicate the continuous layer of telopodes with tiny vesicles bordering the ganglion. A string of glial cell nuclei was present within the left portion. The thick arrow shows the vacuole above the nucleus of the neuron. Four small dark granules were present in the other neurons. Inset: Several vacuoles fill the cytoplasm of the two degenerating neurons (light microscopy and H&E staining); B: 7.4 μm deeper from Figure A. Between the thin arrows there is no continuous layer of telopodes, instead some vesicles are seen. The left portion has disappeared. The remaining portion is part of a pre-apoptotic neuron with a dark cytoplasm and pyknotic nucleus (arrowhead). Above this neuron is the nuclei of normal glial cells. The thick arrow indicates a vacuole in the neuron. Inset: Apoptotic neurons with strongly amphophilic cytoplasm and rest of the pyknotic nuclei (light microscopy; H&E staining); C: 7.8 μm deeper from Figure B. The defect of the telopodes (between the thin arrows) was shorter. Double layer of telopodes below the defect. In the upper part of the ganglion, there is a large neuron with a few small dark, dense dots, whereas the pre-apoptotic neuron from the middle of the ganglion is no longer present; D: 4.7 μm deeper from Figure C. There is a continuous layer of telopodes (between arrows) with a “remnant” of the double layer, as shown in Figure C. The dark granules in the neurons were larger and more numerous. Arrowhead shows a neuron with both dark granules and vacuoles. Inset: The cytoplasm of the neurons was filled with diastase-resistant PAS+ lipofuscin granules (light microscopy; PAS-D staining). The scale bar (50 µm) applies to all the subfigures. Ggl: Ganglion; H&E: Hematoxylin & eosin; n: Neuron; PAS: Periodic acid-Schiff; PAS-D: Periodic acid-Schiff with diastase; SMC: Smooth muscle cells.
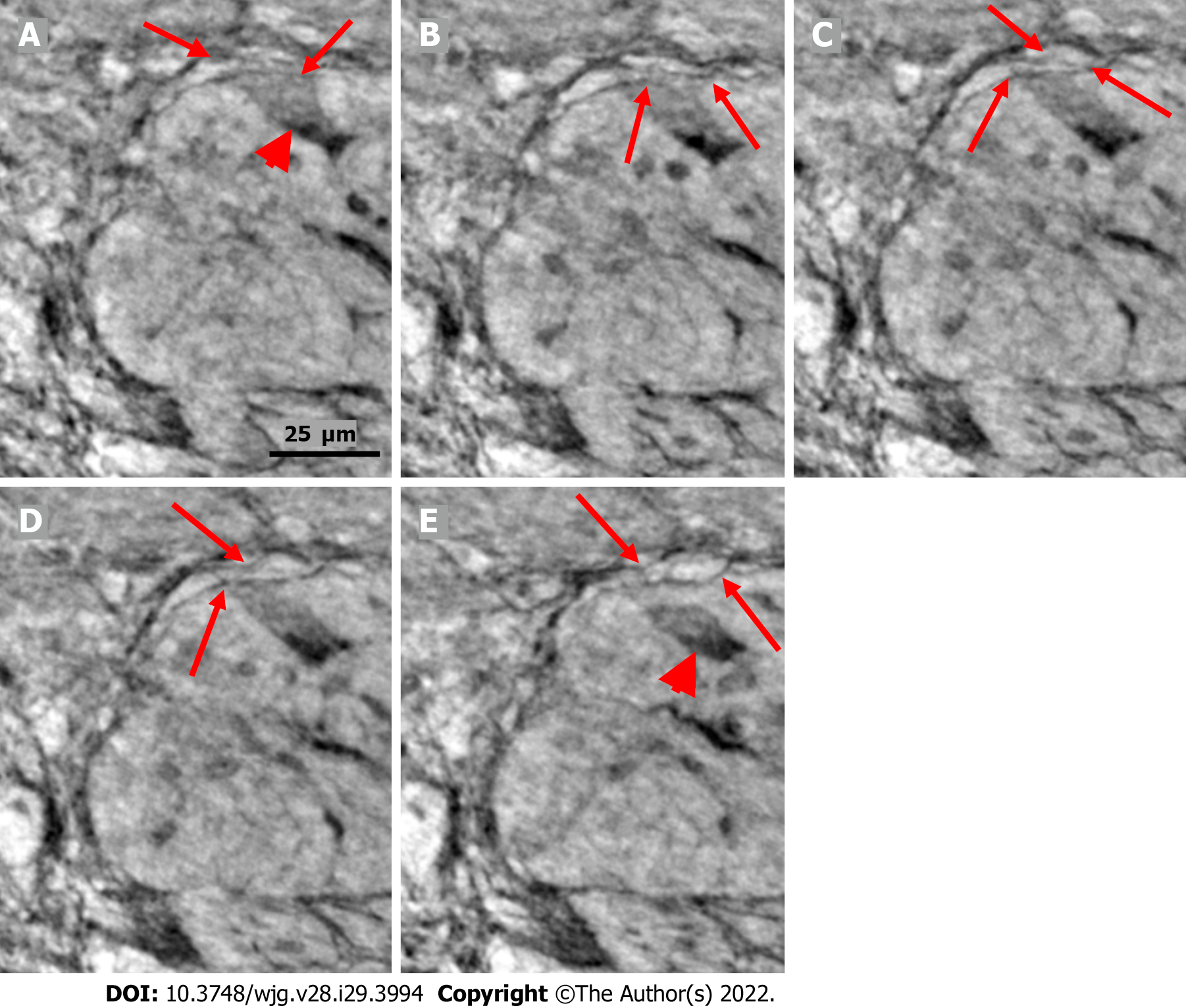
Figure 6 Series of virtual slices covering 6.
4 μm thickness of the ganglion from one patient. A: Neuron with shrunken pyknotic nucleus and dark cytoplasm containing vacuoles at the edge of the ganglion (arrowhead). A part of the cytoplasm of the pre-apoptotic neuron is present between the telopodes (arrows); B: 2.0 μm deeper from Figure A. The lower telopode was discontinuous (arrows); C: 0.7 μm deeper from Figure B. The two telopodes are intact, and almost all of the cytoplasm between them has disappeared; D: 0.7 μm deeper from Figure C. Pre-apoptotic neurons seem to be in contact with the lower telopode; E: 3.0 μm deeper from Figure D. The pre-apoptotic neuron (arrowhead) lies within the ganglion, without contact with the telopodes. Arrows point to two telopodes on the figures. The scale bar (25 μm) applies to all the subfigures.
- Citation: Veress B, Peruzzi N, Eckermann M, Frohn J, Salditt T, Bech M, Ohlsson B. Structure of the myenteric plexus in normal and diseased human ileum analyzed by X-ray virtual histology slices. World J Gastroenterol 2022; 28(29): 3994-4006
- URL: https://www.wjgnet.com/1007-9327/full/v28/i29/3994.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i29.3994