©The Author(s) 2022.
World J Gastroenterol. May 7, 2022; 28(17): 1751-1767
Published online May 7, 2022. doi: 10.3748/wjg.v28.i17.1751
Published online May 7, 2022. doi: 10.3748/wjg.v28.i17.1751
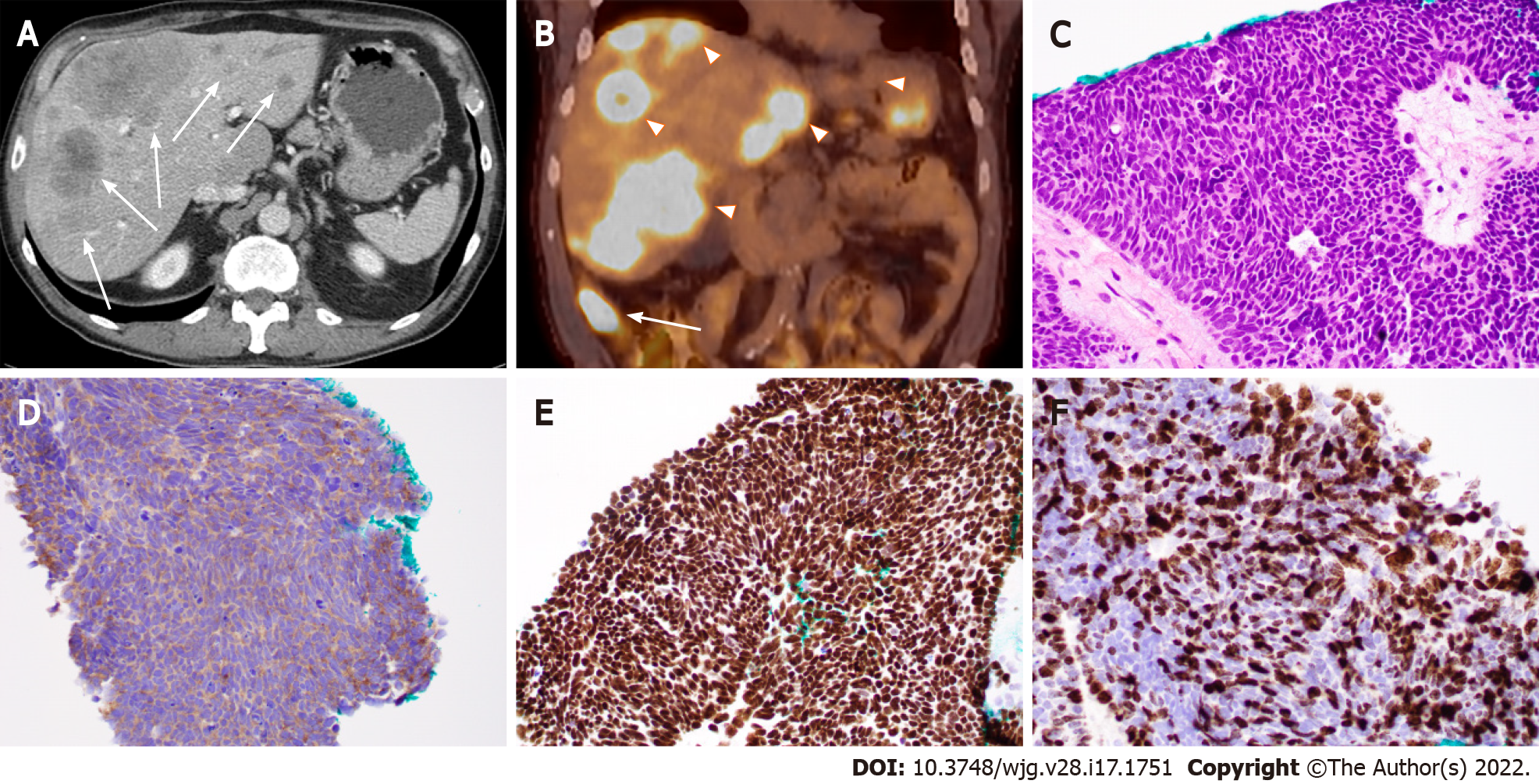
Figure 1 Ileal small cell neuroendocrine carcinoma with liver metastases in a 71-year-old man.
A: Computed tomography (CT) image (axial) showing ileal and multifocal liver lesions (arrow); B: Positron emission tomography-CT image showing ileal (arrow) and liver lesions (arrowheads) with hypermetabolic activity; C: Histopathologic features of small cell neuroendocrine carcinoma. Note the tumor cells with peripheral palisading, rosetting, scant cytoplasm, nuclear molding, finely granular chromatin, and lack of prominent nucleoli; D-F: Tumor cells with positive immunoreactivity for synaptophysin (D) and CDX2 (E) as well as a high Ki-67 proliferation index (70%) (F). (C-F: 400 ×).
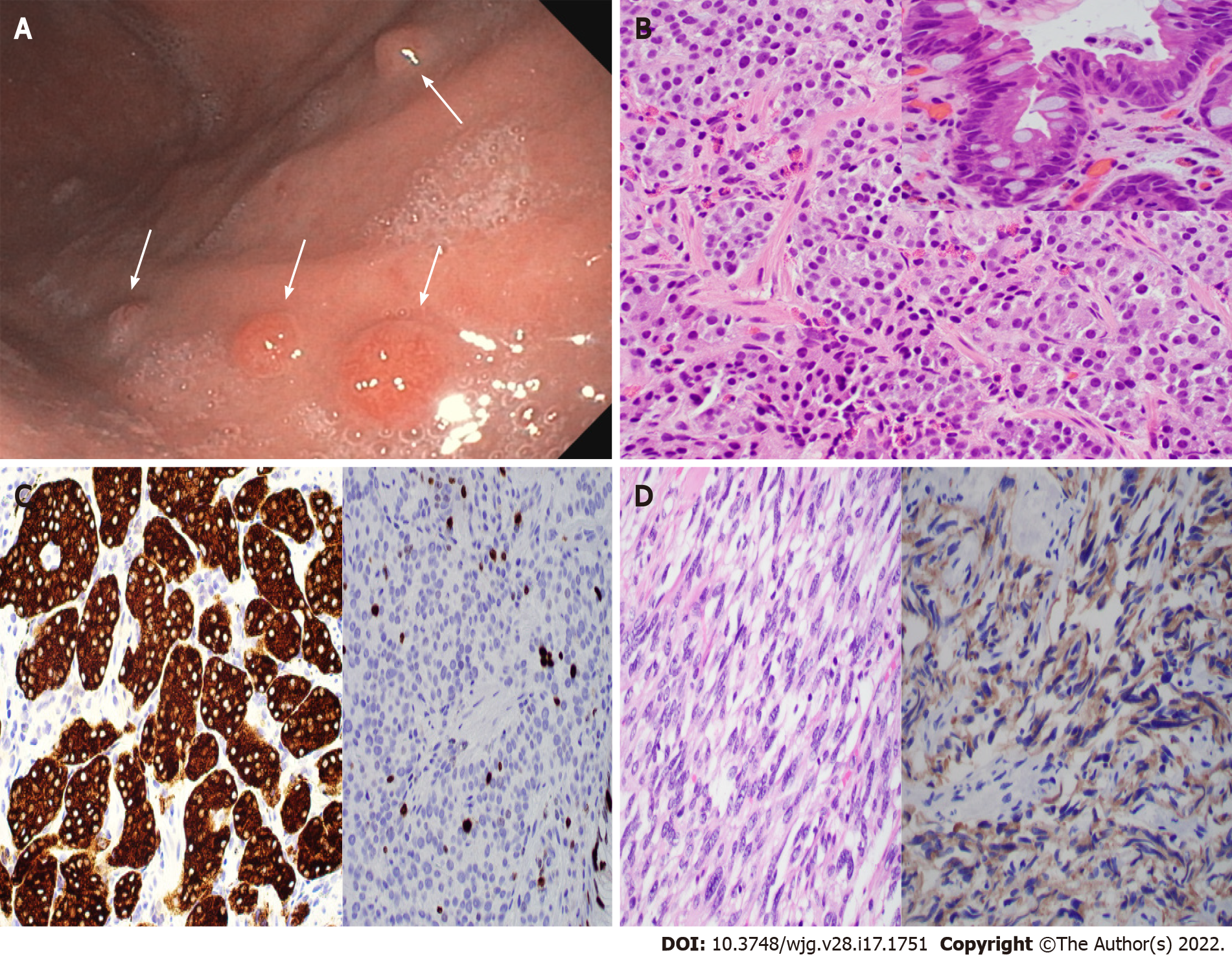
Figure 2 Type 1 gastric neuroendocrine tumor with usual and unusual histology.
A: Endoscopic photo showing four nodules (arrows) in the gastric body of a 56-year-old woman with a history of autoimmune chronic atrophic gastritis (ACAG); B and C: Biopsy of the nodules showing well-differentiated neuroendocrine tumor (B, H&E stain, 400 ×) with intestinal metaplasia on the overlying mucosa (B inset), positivity for synaptophysin (C, left), and a 7% Ki67 index (C, right); D: Gastric biopsy in an 83-year-old woman with ACAG showing a neuroendocrine tumor with unusual spindle cell morphology mimicking gastrointestinal stromal tumor (GIST) (left, H&E stain), which is positive for synaptophysin (right) and negative for GIST markers.
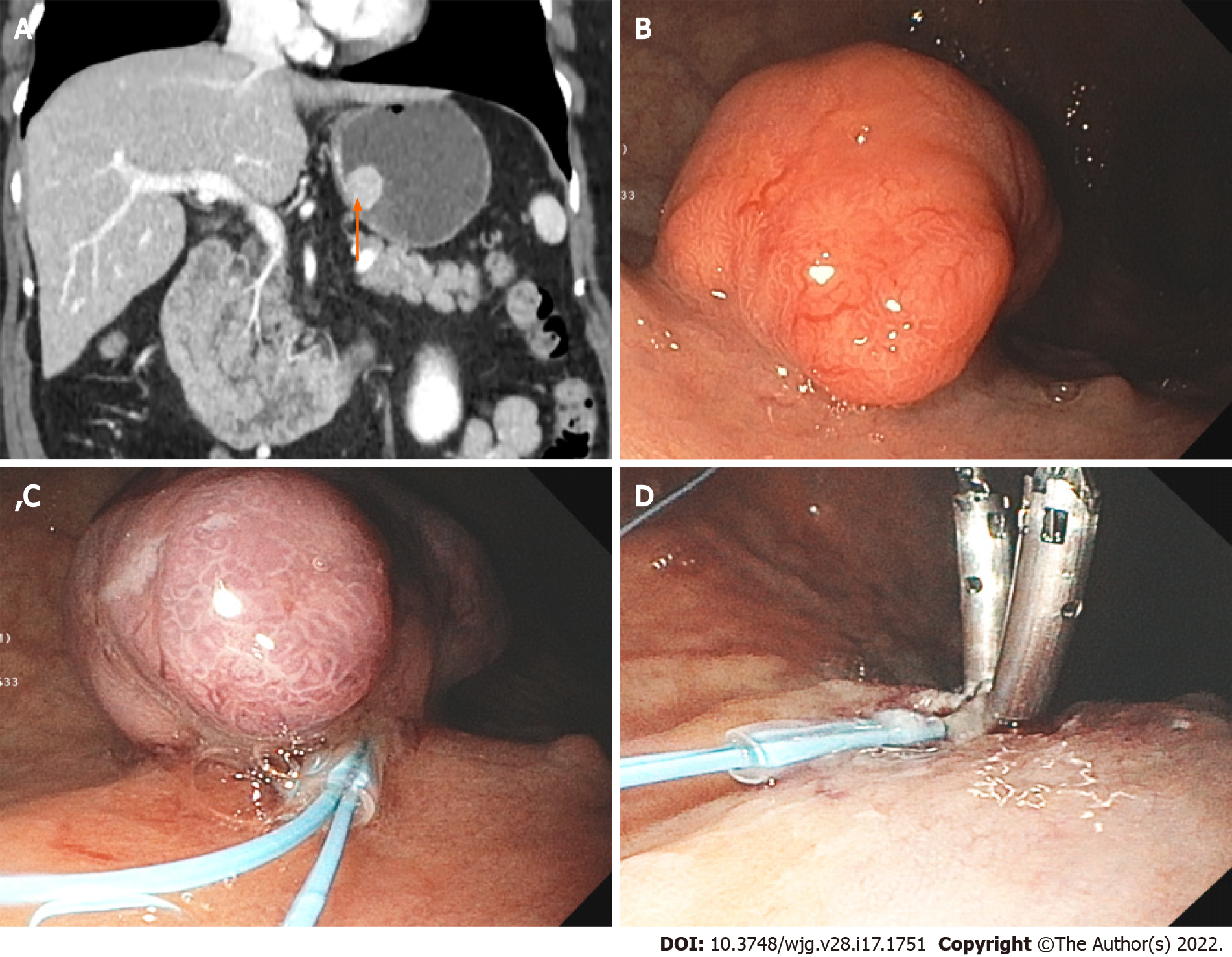
Figure 3 Large gastric neuroendocrine tumor.
A: Computed tomography image showing a 2.3 cm mass (arrow) at the posterior wall of the body of a 74-year-old woman with a history of autoimmune chronic atrophic gastritis; B: Endoscopic image showing the large polypoid lesion in the gastric body; C and D: Endoscopic mucosal resection of the polypoid type 1 neuroendocrine tumor.
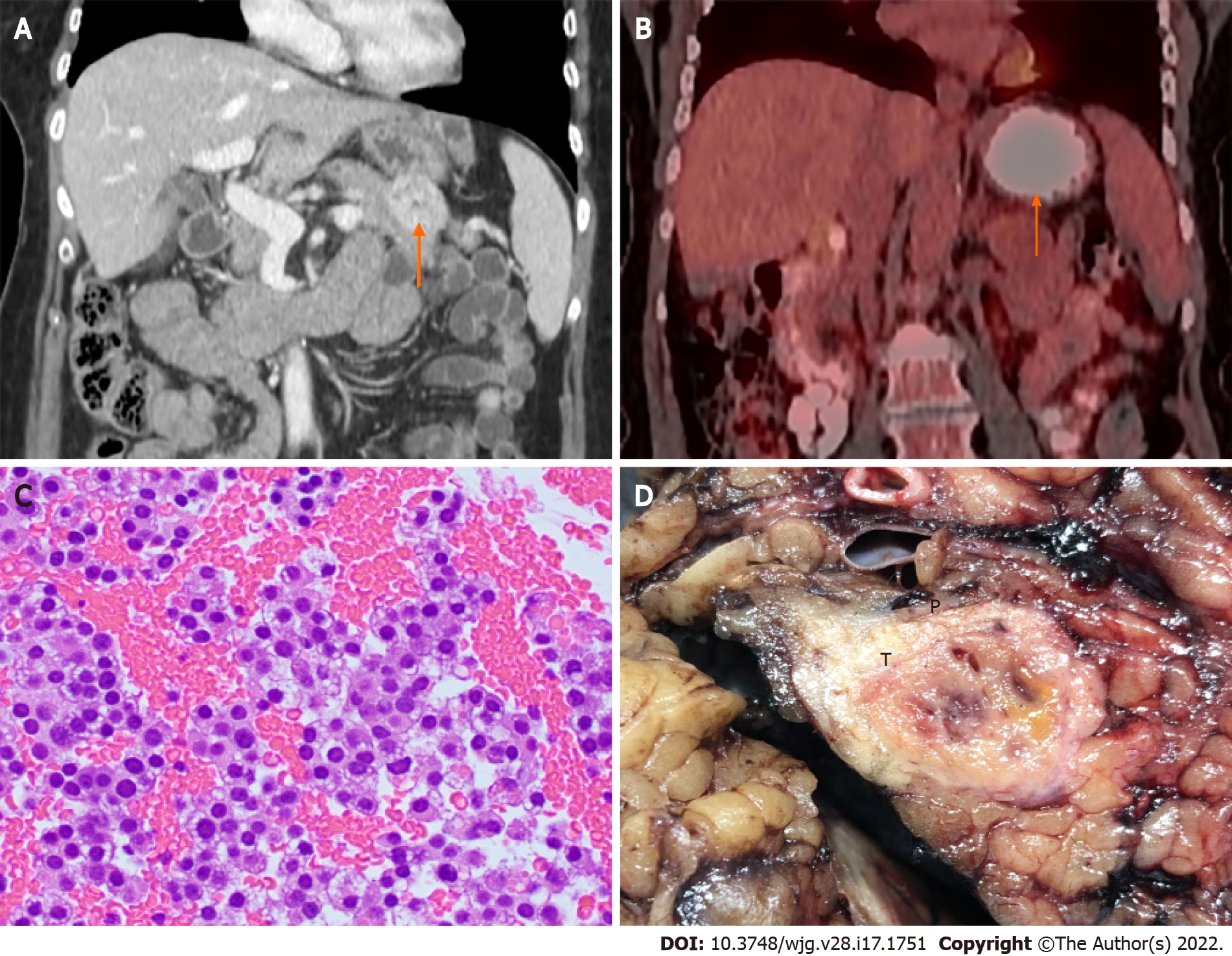
Figure 4 Pancreatic neuroendocrine tumor.
A: Computed tomography (CT) image showing a 3.5 cm distal pancreatic mass (arrow); B: Positron emission tomography-CT image showing a pancreatic mass with hypermetabolic activity (SUVmax = 4.3) (arrow); C: Endoscopic ultrasound-guided fine-needle aspiration showing clusters of neuroendocrine tumor cells with round nuclei and fine stippled “salt-and-pepper” chromatin (H&E stain, 400 ×); D: Distal pancreatectomy showing the gross cut surface of a firm fibrotic pancreatic neuroendocrine tumor (T) with focal hemorrhage.
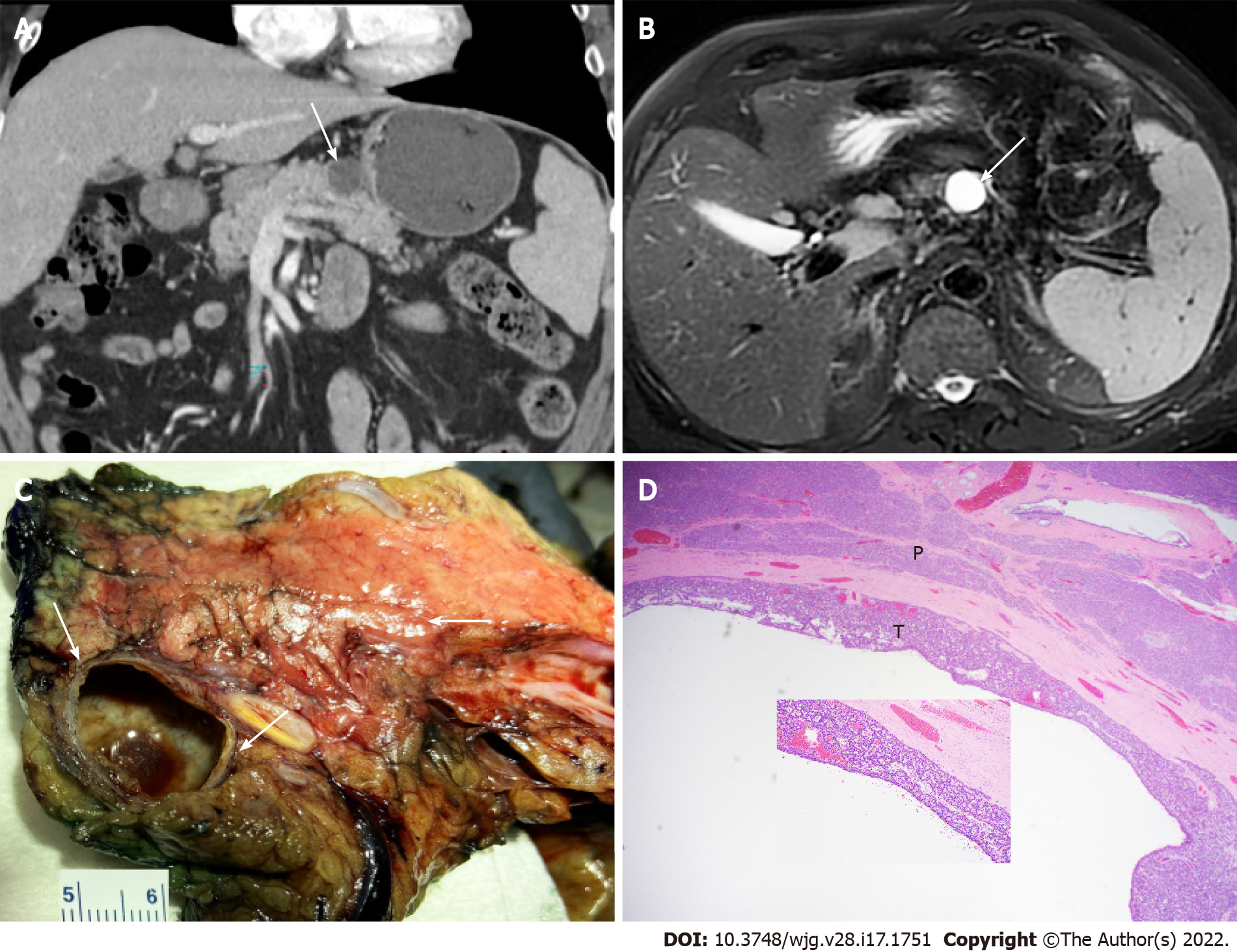
Figure 5 Pancreatic cystic neuroendocrine tumor mimicking mucinous cystic neoplasm.
A: Computed tomography image (axial) showing a 2.2 cm cystic lesion (arrow) in the pancreatic body of a 68-year-old man; B: Magnetic resonance imaging (T2, axial) showing the cystic mass (arrow); C: Partial pancreatectomy specimen showing cut surface of the thin-walled cystic pancreatic neuroendocrine tumor mimicking mucinous cystic neoplasm with no communication with the pancreatic duct (arrows); D: Histopathologic features of cystic pancreatic neuroendocrine tumor with acinar and trabecular architecture (T) and surrounding pancreatic parenchyma (P) (H&E stain, 40 ×, inset 200 ×).
- Citation: Yin F, Wu ZH, Lai JP. New insights in diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. World J Gastroenterol 2022; 28(17): 1751-1767
- URL: https://www.wjgnet.com/1007-9327/full/v28/i17/1751.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i17.1751