©The Author(s) 2021.
World J Gastroenterol. May 28, 2021; 27(20): 2434-2457
Published online May 28, 2021. doi: 10.3748/wjg.v27.i20.2434
Published online May 28, 2021. doi: 10.3748/wjg.v27.i20.2434
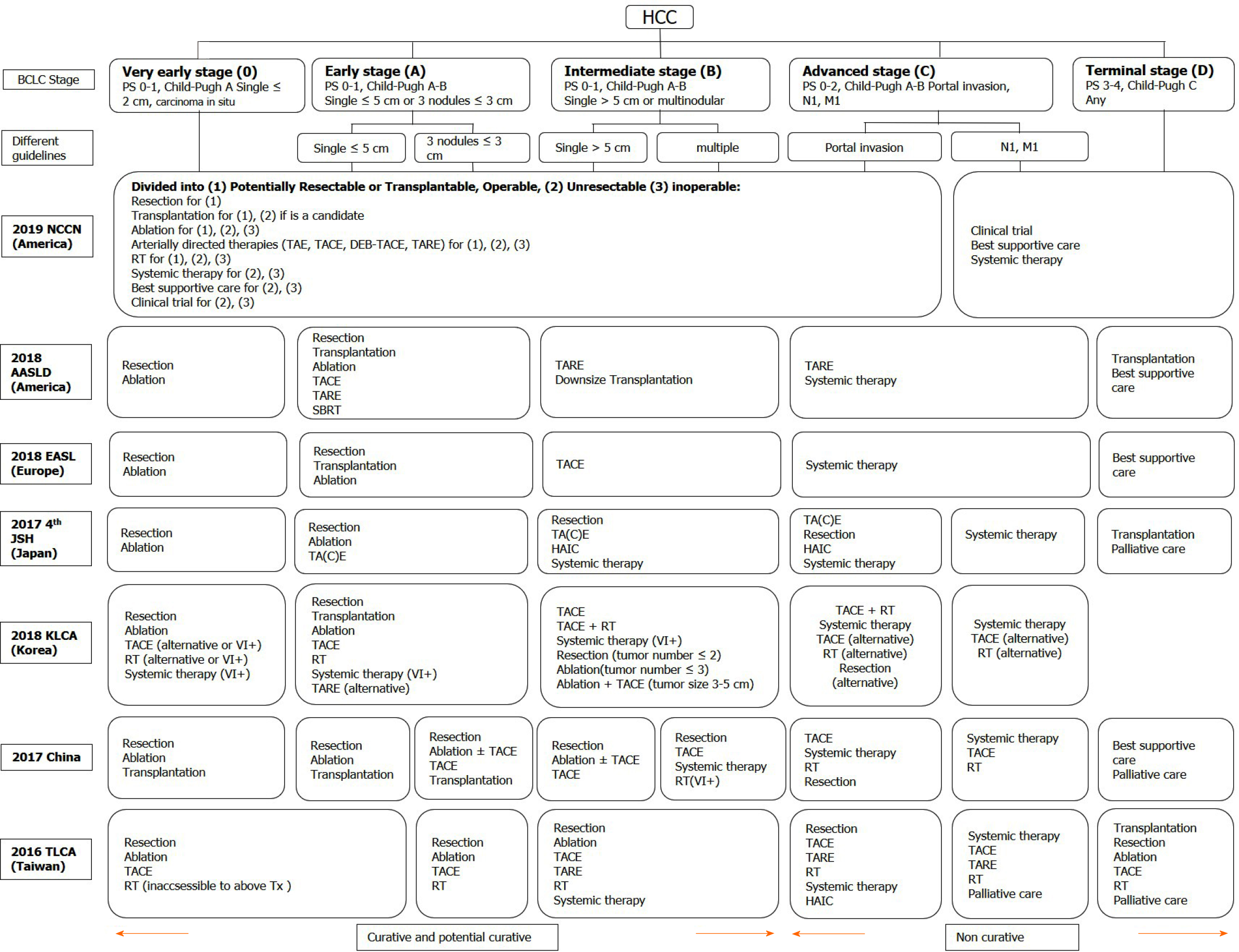
Figure 1 Treatment recommendations modified in different guidelines according to the Barcelona clinic liver cancer stage.
HCC: Hepatocellular carcinoma; BCLC: Barcelona clinic liver cancer; PS: Performance status; TA(C)E: Trans-arterial (chemo)embolization; TARE: Trans-arterial radioembolization; DEB-TACE: Trans-arterial chemoembolisation with drug-eluting beads; RT: Radiotherapy; SBRT: Stereotactic body radiation therapy; HAIC: Hepatic arterial infusion chemotherapy; NCCN: National Comprehensive Cancer Network; AASLD: American Association for the Study of Liver Diseases; EASL: European Association for the Study of the Liver; JSH: Japan Society of Hepatology; KLCA: Korean Liver Cancer Association; TLCA: Taiwan Liver Cancer Association; Tx: Treatments; VI+: Positive vascular or bile duct invasion.
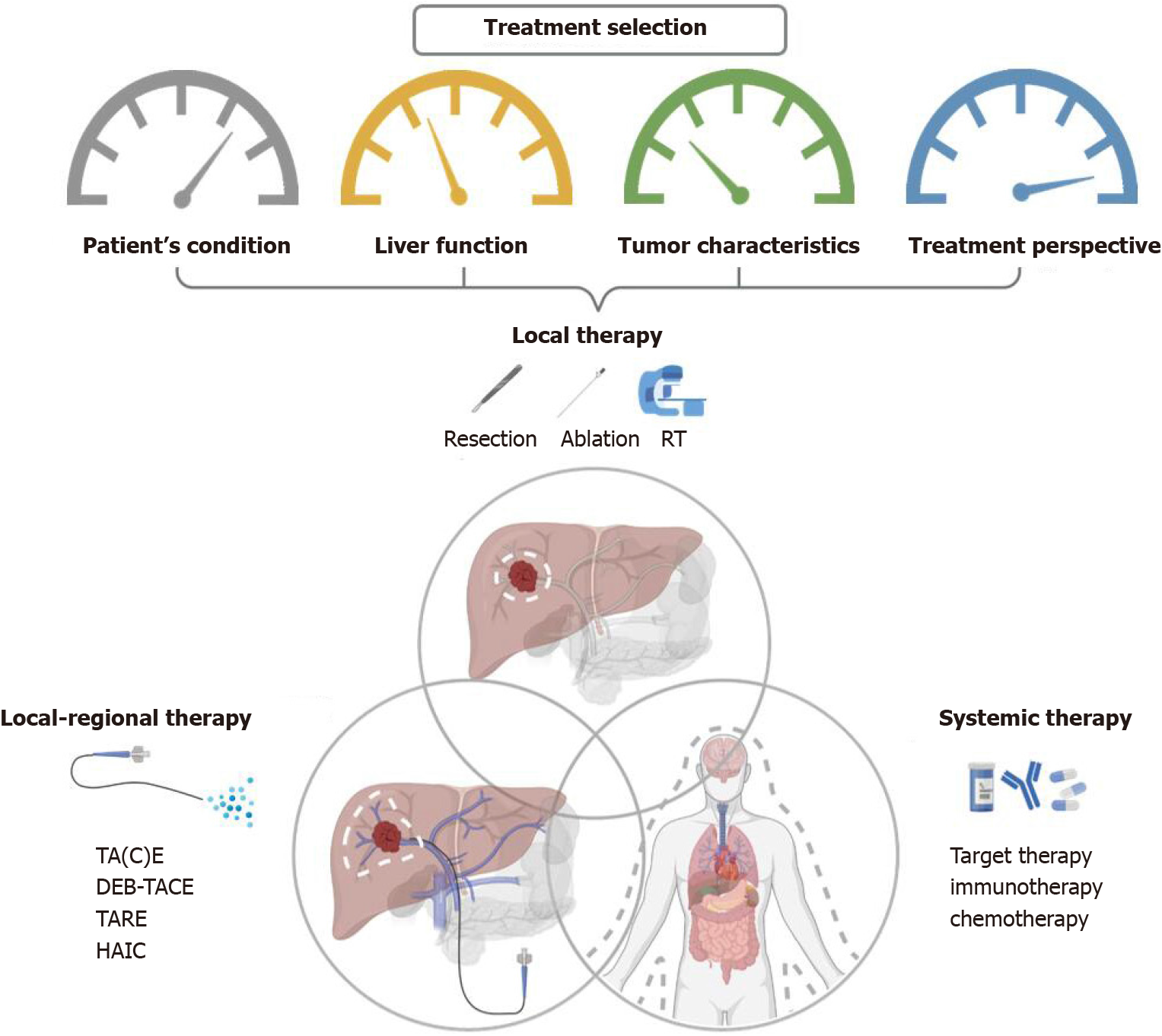
Figure 2 Overview of therapeutic options and the consideration behind the combination of different combined modalities for liver cancer.
RT: Radiotherapy; TA(C)E: Trans-arterial (chemo)embolization; TARE: Trans-arterial radioembolization; DEB-TACE: Trans-arterial chemoembolisation with drug-eluting beads; HAIC: Hepatic arterial infusion chemotherapy.
Figure 3 Current treatment recommendations based on the Barcelona clinic liver cancer stage and the potential roles of radiotherapy.
HCC: Hepatocellular carcinoma; BCLC: Barcelona clinic liver cancer; PS: Performance status; TACE: trans-arterial chemoembolization; TARE: Trans-arterial radioembolization; HAIC: Hepatic arterial infusion chemotherapy; RT: Radiotherapy; CFRT: Conventionally fractionated radiotherapy; HFRT: Hypo-fractionated radiotherapy; SBRT: Stereotactic body radiation therapy; PVT: Portal vein thrombosis; STAR effect: Systemic therapy augmented by radiotherapy effect.
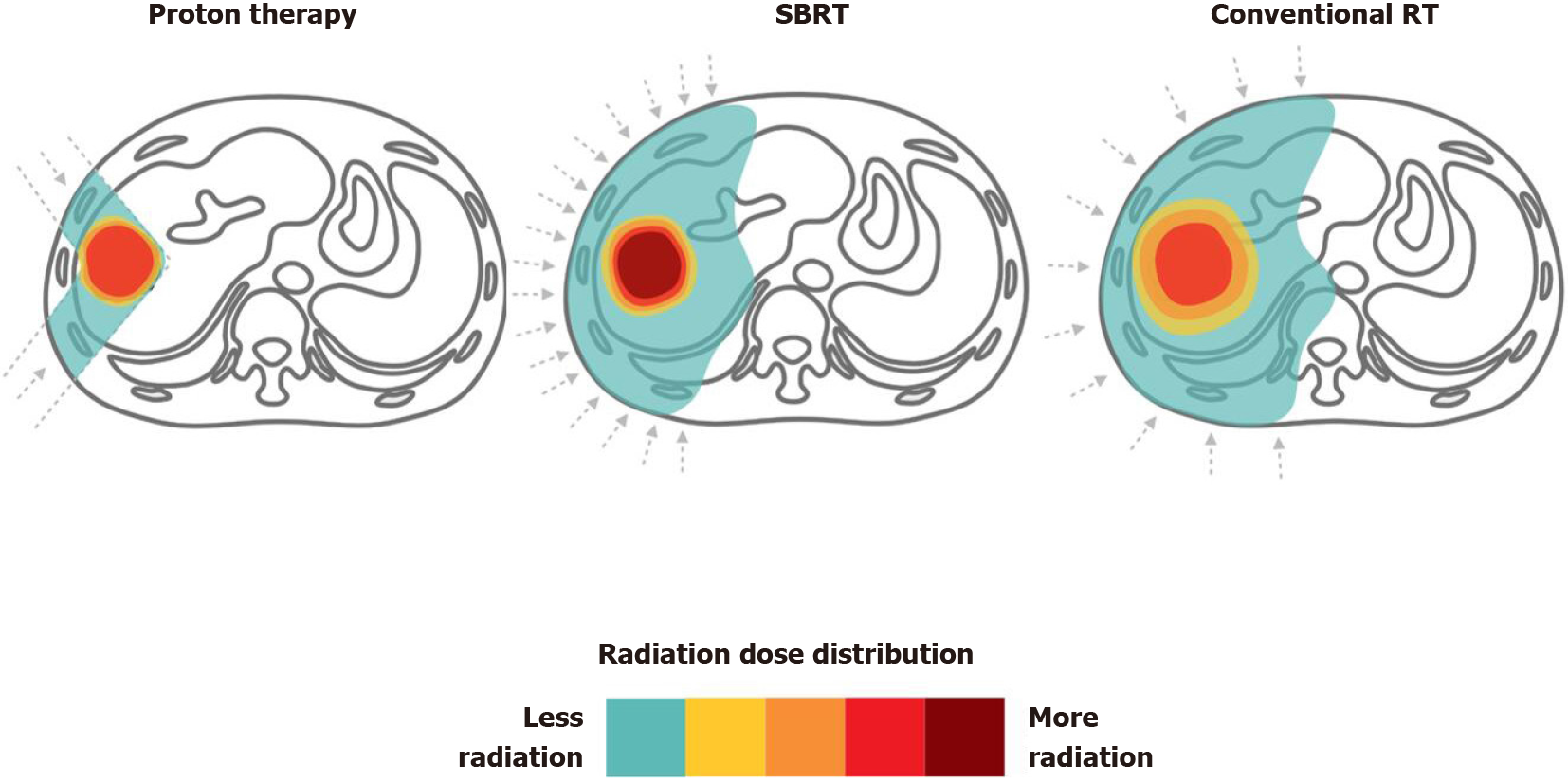
Figure 4 Proton therapy vs stereotactic body radiation therapy vs conventional radiotherapy for liver tumors.
Dose distributions for a proton (left), stereotactic body radiation therapy (middle) and conventional radiotherapy (right) hepatocellular carcinoma radiotherapy plan are illustrated for comparison. RT: Radiotherapy; SBRT: Stereotactic body radiation therapy.
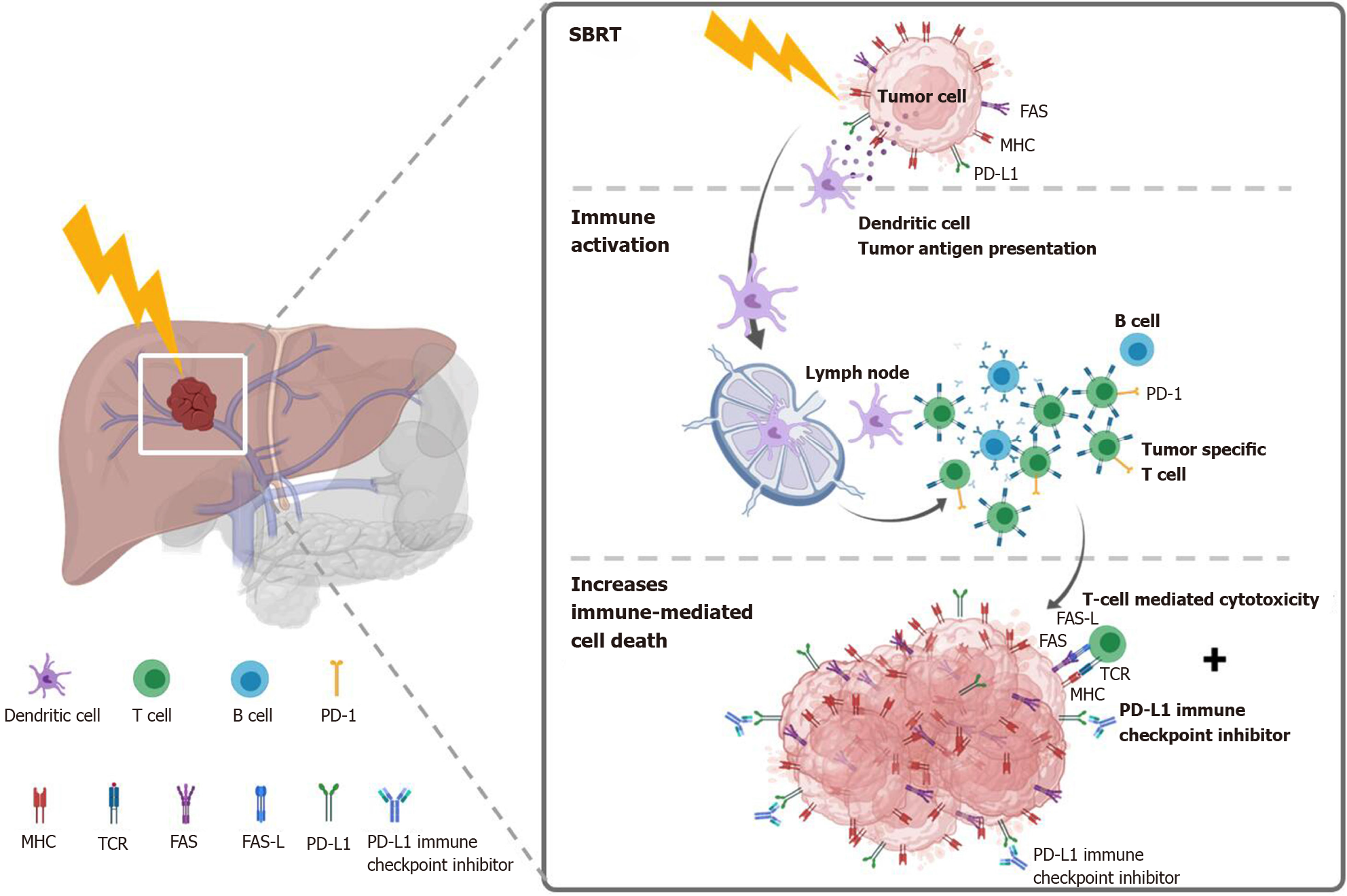
Figure 5 Potential mechanism of stereotactic body radiation therapy combined with systemic therapy to induce the systemic therapy augmented by radiotherapy effect (also known as immunotherapy and stereotactic ablative radiotherapy) for liver tumors.
Stereotactic body radiation therapy (SBRT) induces antigen release and immunogenic cell death, activation of several transcription factors and signal pathways, as well as dendritic cell antigen presentation and maturation, resulting in proliferation of tumor-specific T cells and immune-mediated cytotoxicity. SBRT combined with Immune-checkpoint inhibitors augmented the tumoricidal effect by upregulates major histocompatibility complex and FAS on tumor cells, increasing susceptibility to T-cell-mediated cell death. MHC: Major histocompatibility complex; TCR: T cell receptor; FAS-L: FAS ligand.
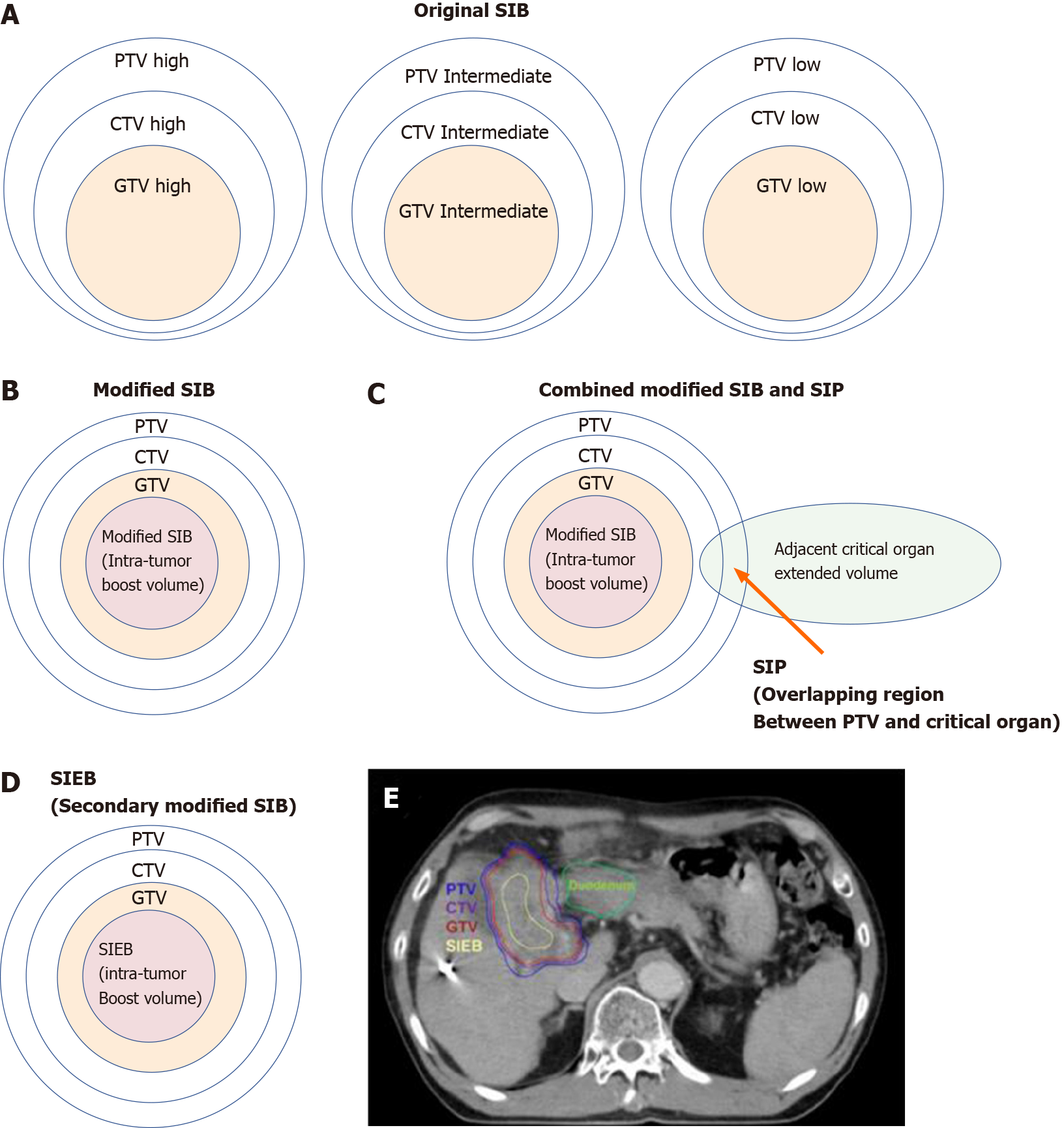
Figure 6 Cartoon and case illustrations of simultaneously integrated boost, modified simultaneously integrated boost, combined simultaneously integrated boost and simultaneously integrated protection, and simultaneously integrated inner-escalated boost dose-prescription techniques.
A: A simple cartoon figure representing original simultaneously integrated boost (SIB) dose prescription. Original SIB is prescribed in different doses per fraction to different target regions according to the risk levels of recurrence. For example, uniform doses per fraction may be given at planning target volume (PTV) high, intermediate, and low with 240 centigray (cGy) (simultaneously integrated boost), 180-200 cGy (traditional dose per fraction), and 160 cGy (inferior to the traditional dose) in the same fractions, respectively; B: A simple cartoon figure representing a modified SIB dose prescription. Traditionally, radiotherapy is prescribed as a uniform dose per fraction (e.g., 200 cGy) on PTV, which provides a homogeneous dose to cover clinical target volume (CTV) and gross target volume (GTV). Recently, to maximally enhance the possibility of tumor control, a modified SIB technique is applied using a planned non-homologous dose distribution, i.e., escalating a simultaneous intra-tumor boost (e.g., 220-240 cGy per fraction) in addition to a traditional covering dose to the PTV (e.g., 200 cGy per fraction) in the same treatment fractions; C: A simple cartoon figure representing combined modified SIB and simultaneously integrated protection (SIP) dose prescription. To reduce treatment toxicities to adjacent critical organs/tissue, SIP was developed in conjunction with modified SIB. SIP prescribes an inferior-to-traditional dose per fraction, e.g., 150 cGy, to the overlapping region between the PTV and the extended critical organ volume (as shown by the long arrow); D: A simple cartoon figure representing simultaneously integrated inner-escalated boost (SIEB) dose prescription. For further enhanced therapeutic gain (i.e., increased tumor control and decreased treatment toxicity) in managing patients with unresectable liver tumors, we applied a secondary modified SIB (also termed SIEB). SIEB further escalates the intra-tumor boost (e.g., 240-260 cGy per fraction) based on a planned generally attenuated peri-tumor dose (e.g., 120-150 cGy per fraction delivered to the PTV), administered simultaneously. The intra-tumor SIEB boost volume is delineated as a uniform-inner-shrinkage area from the GTV with a margin of 1-10 mm (i.e., a geometrically central zone), depending on the tumor size, the intensity of the dose escalation, the level of liver preservation, the closeness of the gastrointestinal organs to the irradiation targets, and the patient's condition. Note that an additional most-inner SIEB boost volume with the highest dose per fraction (e.g., 260-300 cGy or higher) might be considered for highly selected patients with very bulky tumors. E: A clinical case with SIEB dose prescription. The blue, purple, and red lines show the PTV, CTV, and GTV of the irradiating target, respectively. In this case, based on the physician’s choice and the patient’s condition, a dose per fraction of 120 cGy was prescribed to the PTV, 150 cGy to the CTV, and 200 cGy to the GTV. Finally, in the yellow-outlined region, 280 cGy per fraction was simultaneously delivered to the SIEB boost volume. A total of 30 fractions were given, generating total doses levels of 3600 cGy, 4500 cGy, 6000 cGy, and 7400 cGy to the PTC, CTV, GTV, and SIEB boost volume, respectively. Note that the most peripheral dose per fraction of 120 cGy was chosen mainly due to a very close distance between the PTV and an adjacent critical organ, i.e., the duodenum. This short-distance closeness could easily lead irradiation to harm the duodenum under the context of daily organ motion. SIB: Simultaneously integrated boost; SIP: Simultaneously integrated protection; SIEB: Simultaneously integrated inner-escalated boost; PTV: Planning target volume; CTV: Clinical target volume; GTV: Gross target volume.
- Citation: Chen LC, Lin HY, Hung SK, Chiou WY, Lee MS. Role of modern radiotherapy in managing patients with hepatocellular carcinoma. World J Gastroenterol 2021; 27(20): 2434-2457
- URL: https://www.wjgnet.com/1007-9327/full/v27/i20/2434.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i20.2434