©The Author(s) 2020.
World J Gastroenterol. Aug 28, 2020; 26(32): 4802-4816
Published online Aug 28, 2020. doi: 10.3748/wjg.v26.i32.4802
Published online Aug 28, 2020. doi: 10.3748/wjg.v26.i32.4802
Figure 1 Interleukin 2 receptor subunit gamma knockout Syrian hamsters (ZZU001) generated by CRISPR/Cas9.
A: Schematic representation of the Syrian hamster interleukin 2 receptor subunit gamma (IL2RG) gene. The single-guide RNA target sequence is shown in green with the protospacer adjacent motif shown in red; B: Polymerase chain reaction product sequencing confirming the founder is shown. “Stat” refers to the mutant site; C: Sequencing assay for single-guide RNA/Cas9-IL2RG-induced mutation in the target region in Syrian hamsters. Multiple deletions are depicted by dashes; D: Quantitative real-time polymerase chain reaction analysis of IL2RG mRNA expression in the thymus of wild type and ZZU001 Syrian hamsters. bP < 0.01. n = 3. Glyceraldehyde 3-phosphate dehydrogenase mRNA expression was used as an internal control; E: Western blotting assay to compare the expression of IL2RG protein in wild type and ZZU001 Syrian hamsters. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. KO: Knock-out; sgRNA: Single-guide ribonucleic acid; WT: Wild type.
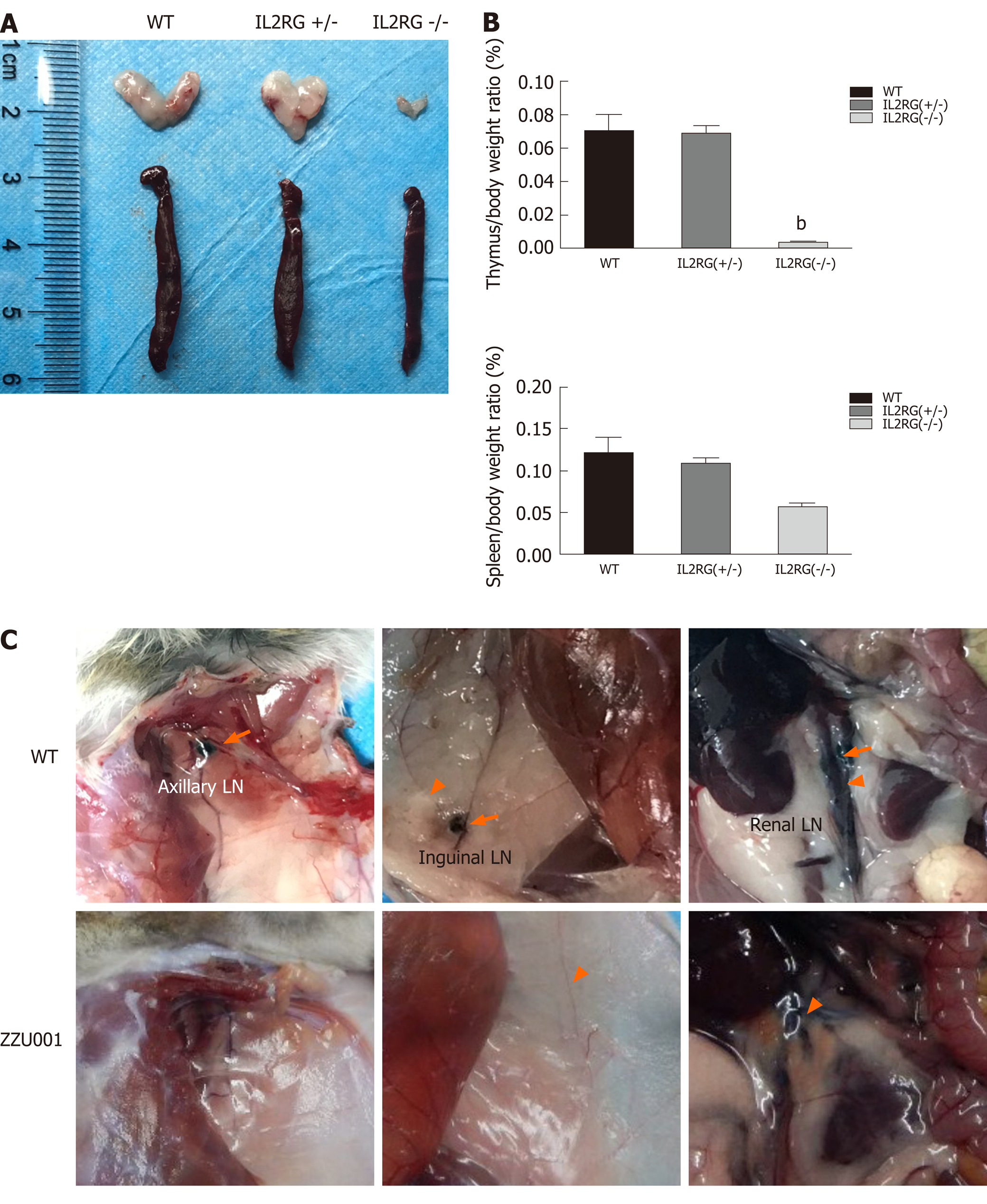
Figure 2 ZZU001 Syrian hamsters demonstrate abnormal lymphoid cell development and impaired immune function.
A: The thymus and spleen of interleukin 2 receptor subunit gamma (IL2RG) knock-out (KO) hamsters were both evidently smaller than those of age-matched interleukin 2 receptor subunit gamma +/- and wild type (WT) hamsters; B: Comparison of spleen-to-body weight ratio. Each value represents the mean ± standard deviation (n = 3/group), Student’s t-test. bP < 0.01; and C: Dye labeling of Syrian hamster lymph nodes (LNs) and lymphatic vessels. Efferent lymphatic vessel (orange arrowhead). Axillary LN, Inguinal LN, and Renal LN (orange arrow) are LNs.
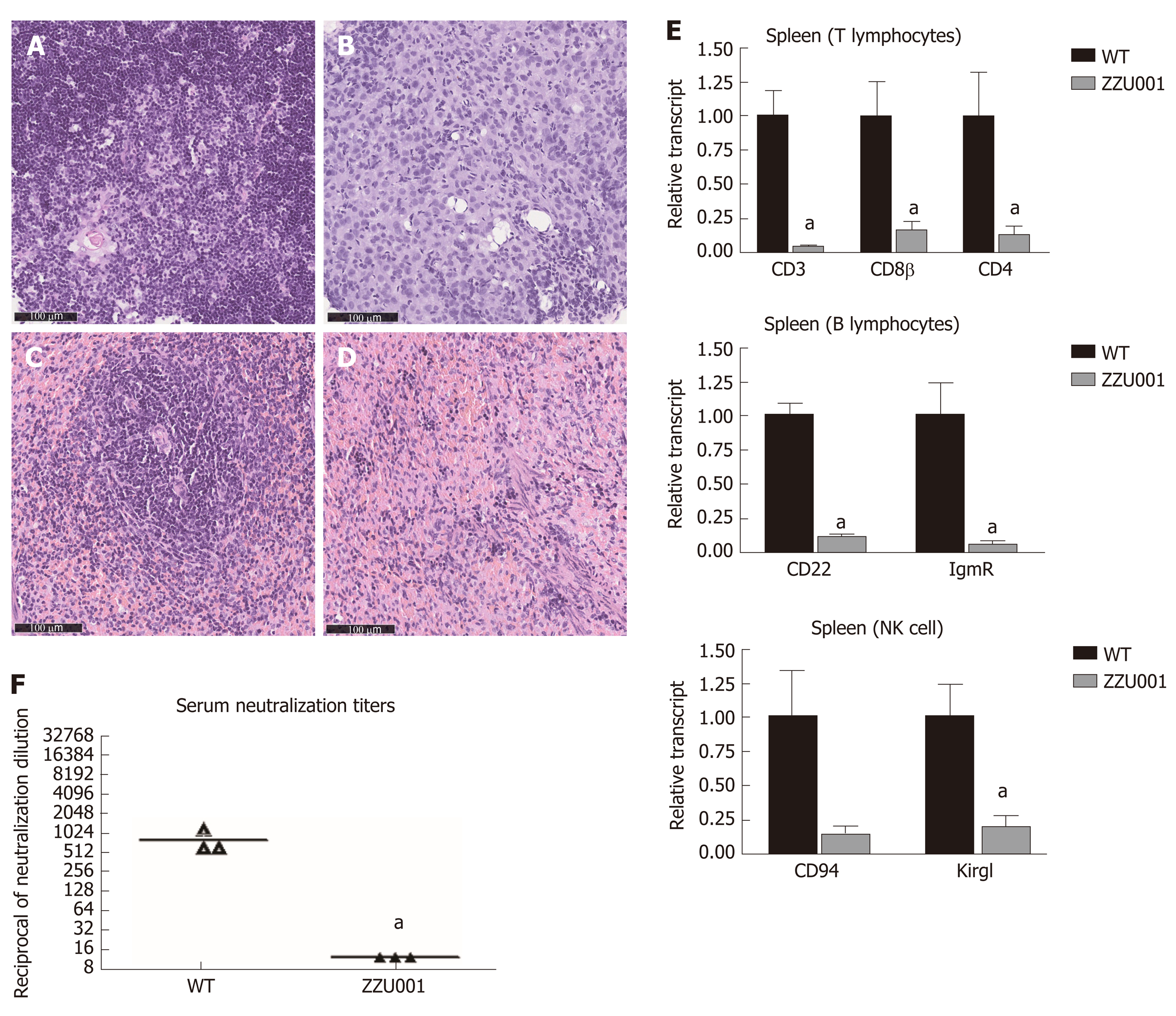
Figure 3 ZZU001 Syrian hamsters demonstrate immune despair cellular abnormalities in lymphoid development.
A and C: Representative histology of the wild type (WT) thymus and spleen. Bars = 100 µm; Representative histology of ZZU001 Syrian hamsters thymus B and spleen D and E: The expression of the indicated lymphocyte-specific genes in spleen homogenates was determined by reverse transcriptase quantitative polymerase chain reaction using the 2^ (-ΔΔCt) method. aP < 0.05. n = 3; F: Development of neutralizing antibodies against AdV-5. n = 3. aP < 0.05.
Figure 4 Xenotransplantation of human pancreatic cancer cells into ZZU001 Syrian hamsters recapitulates human pancreatic cancer metastasis.
A: Growth curve of human MIA-PaCa-2 pancreatic cancer cells in ZZU01 Syrian hamsters and B-NDG mice. MIAPaCa-2 cells were subcutaneously inoculated into the flank of ZUU001 and B-NDG mice (n = 5/group). Tumors were measured twice weekly and the tumor volume estimated. Mean tumor size ± standard error of the mean is shown. ns: no significance; B: A representative hematoxylin and eosin (HE) stained section of a kidney and adrenal gland with metastatic tumor at the end of the experiment in ZZU001 hamsters (56 d) and B-NDG mice (42 d); C: Representative HE staining of a lung metastasis at 22 d, 29 d, 36 d after the injection of MIA-PaCa-2 cells in ZZU001 hamsters and B-NDG mice; D: Representative HE, Masson, smooth muscle actin (SMA) and vimentin staining of a subcutaneous tumor at 42 d after the injection of the MIA-PaCa-2 cells in ZZU001 hamsters and B-NDG mice. Scale bars = 100 µm.
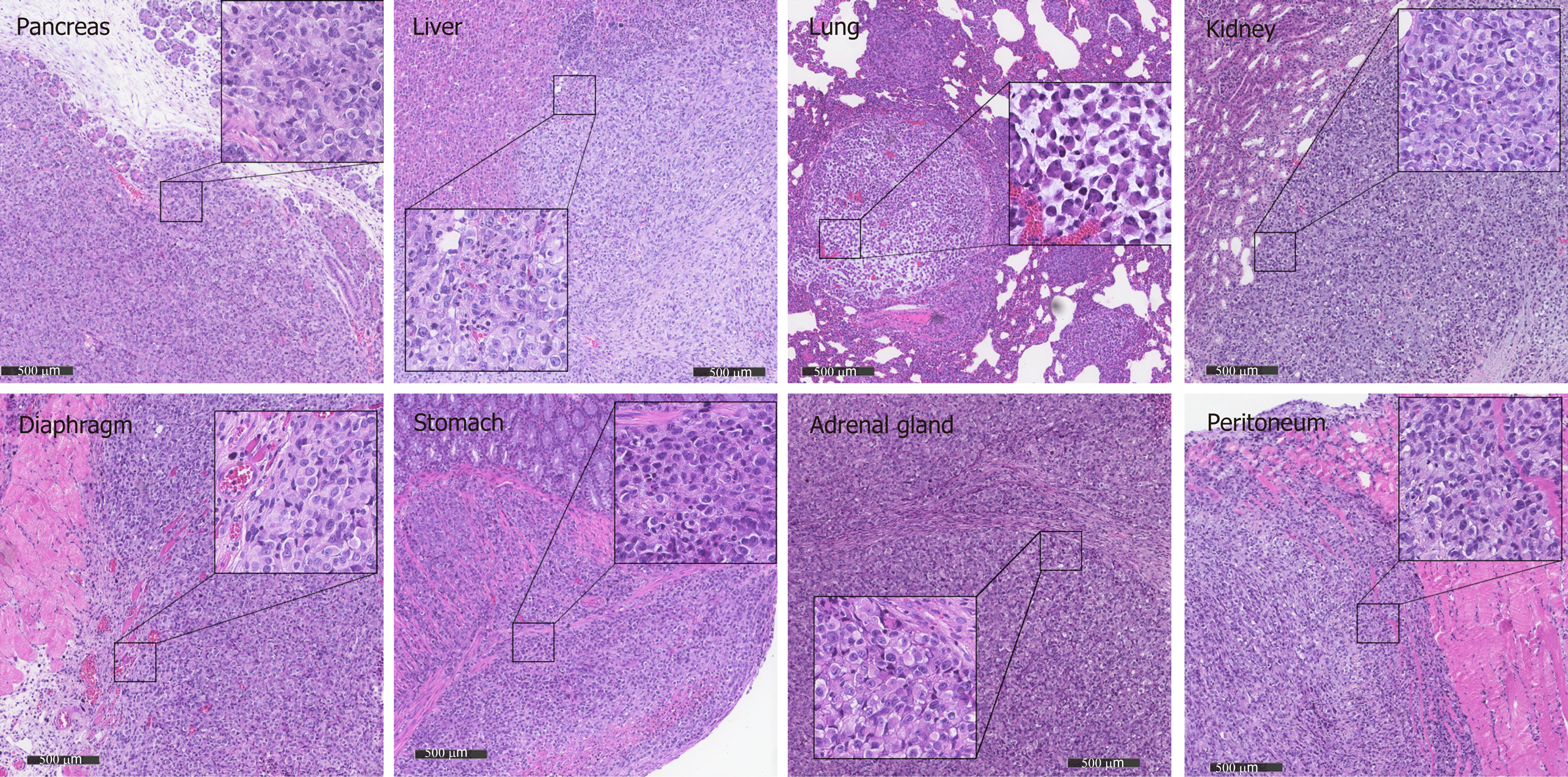
Figure 5 Metastasis in different organs reflects the histology of the primary tumor in ZZZU001 orthotopic xenograft tumor models.
The primary tumors were established by injection of 1 × 106 of MIA-PaCa-2 cells into the pancreatic tail. The representative tumor in the pancreas, liver, lung, kidney, diaphragm, stomach, adrenal gland, and peritoneum of ZZU001 Syrian hamsters are shown. Inset square shows tumor cells at a magnification 400 ×. Scale bars = 500 µm.
- Citation: Miao JX, Wang JY, Li HZ, Guo HR, Dunmall LSC, Zhang ZX, Cheng ZG, Gao DL, Dong JZ, Wang ZD, Wang YH. Promising xenograft animal model recapitulating the features of human pancreatic cancer. World J Gastroenterol 2020; 26(32): 4802-4816
- URL: https://www.wjgnet.com/1007-9327/full/v26/i32/4802.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i32.4802