©The Author(s) 2019.
World J Gastroenterol. Nov 28, 2019; 25(44): 6551-6560
Published online Nov 28, 2019. doi: 10.3748/wjg.v25.i44.6551
Published online Nov 28, 2019. doi: 10.3748/wjg.v25.i44.6551
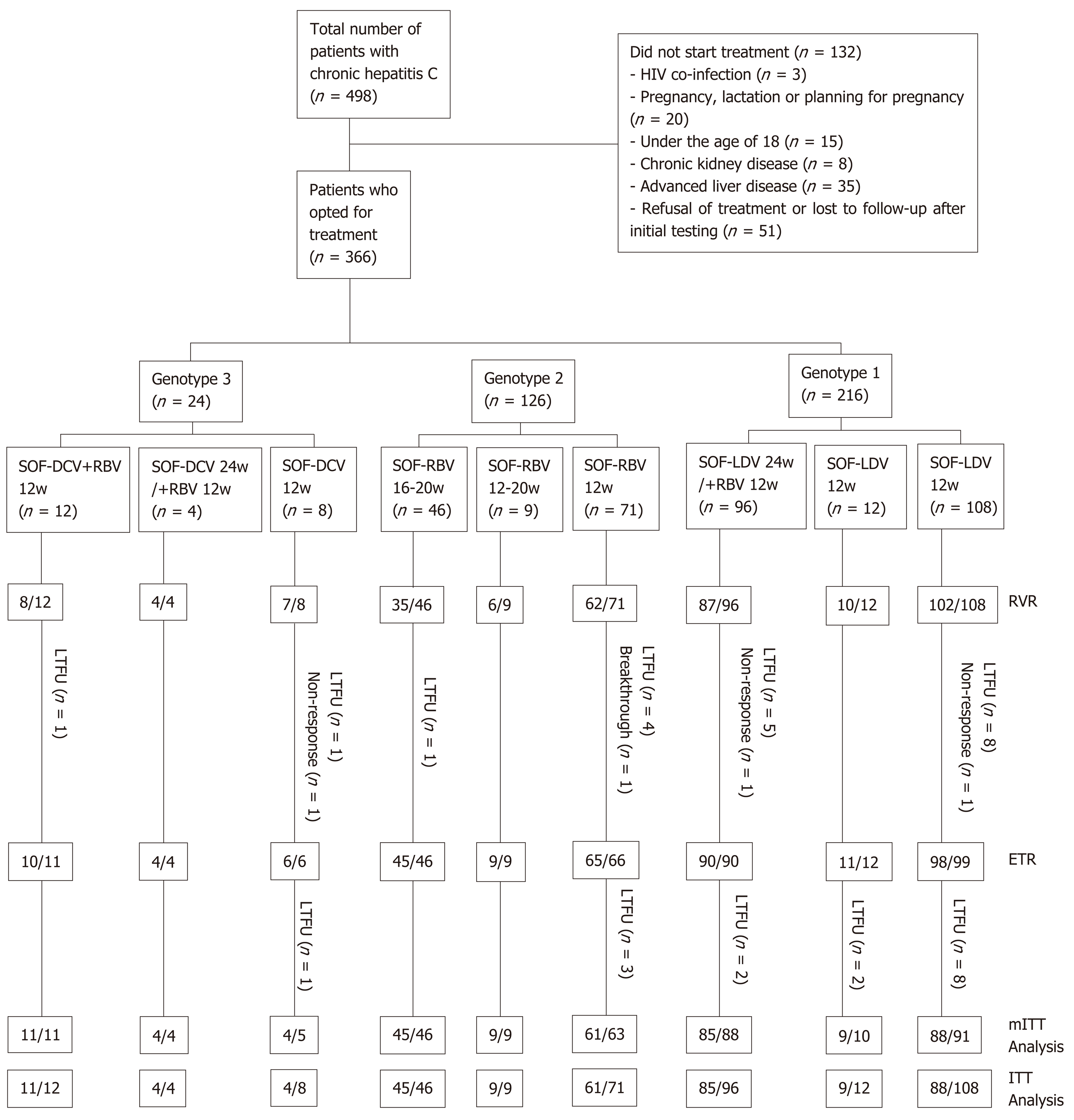
Figure 1 Treatment of chronic hepatitis C virus patients with direct-acting antiviral agents.
RVR: Rapid virological response; ETR: End of treatment response; SVR12: Sustained virological response at post-treatment week 12; ITT: Intention-to-treat; mITT: Modified ITT; SOF: Sofosbuvir; LDV: Ledipasvir; RBV: Ribavirin; DCV: Daclatasvir; mITT: Modified intention-to-treat; LTFU: Lost to follow-up; HIV: Human immunodeficiency virus.
- Citation: Yang Y, Wu FP, Wang WJ, Shi JJ, Li YP, Zhang X, Dang SS. Real life efficacy and safety of direct-acting antiviral therapy for treatment of patients infected with hepatitis C virus genotypes 1, 2 and 3 in northwest China. World J Gastroenterol 2019; 25(44): 6551-6560
- URL: https://www.wjgnet.com/1007-9327/full/v25/i44/6551.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i44.6551