Copyright
©The Author(s) 2018.
World J Gastroenterol. Nov 28, 2018; 24(44): 4962-4973
Published online Nov 28, 2018. doi: 10.3748/wjg.v24.i44.4962
Published online Nov 28, 2018. doi: 10.3748/wjg.v24.i44.4962
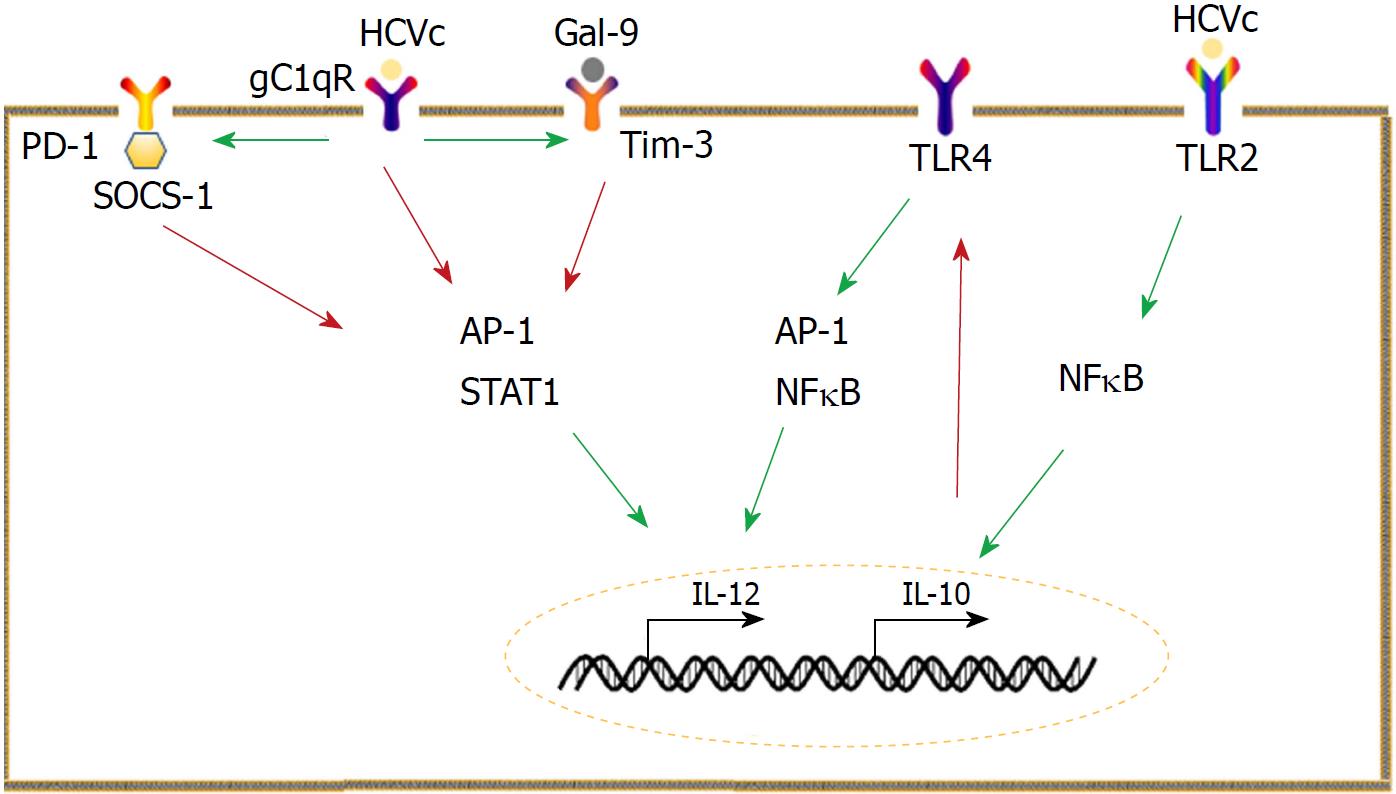
Figure 1 Mechanisms underlying aberrant interleukin-10 and interleukin -12 expression.
Monocytes are a main producer of interleukin-10 (IL-10) in hepatitis C virus (HCV) infection. HCV core protein (HCVc) can stimulate monocytes to produce IL-10, which selectively inhibits Toll-like receptor 4 signaling, leading to impairment of interleukin -12 (IL-12). Programmed cell death 1 (PD-1)/ ligand of PD-1 (PD-L1) signaling and the galectin-9 (Gal-9)/ T cell immunoglobulin and mucin domain 3 (Tim-3) pathways suppress IL-12 production by inhibiting activator protein 1 and signal transducer and activator of transcription 1 activation. The interaction between HCVc and receptor for the globular heads of C1q also inhibits IL-12 production but promotes PD-1/PD-L1 and Gal-9/Tim-3 pathways. The red arrow represents inhibition, whereas the green arrow indicates promotion. HCVc: HCV core protein; IL: Interleukin; TLR: Toll-like receptor; PD-1: Programmed cell death 1; PD-L1: Ligand of PD-1; Gal-9: Galectin-9; TIM-3: T cell immunoglobulin and mucin domain 3; AP-1: Activator protein 1; STAT: Signal transducer and activator of transcription; gC1qR: Receptor for the globular heads of C1q; SOCS: Suppressor of cytokine signaling; NFκB: Nuclear factor-κB.
Figure 2 Role of macrophages in hepatitis C virus infection-associated inflammation.
During hepatitis C virus (HCV) infection, macrophages are the main source of the proinflammatory cytokines [interleukin (IL)-1β, IL-18, tumor necrosis factor (TNF)-α, and IL-6] and the anti-inflammatory IL-10. The production of IL-1β and IL-18 requires two signals, which are initiated by the uptake of intact HCV particles. Signal 1: Following dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin-mediated endocytosis, the HCV genome is released into cytoplasm and the uridine-rich HCV RNA is recognized by endosomal Toll-like receptor 7 (TLR7). This recognition leads to pro-IL-1β and IL-18 production in a myeloid differentiation primary response gene 88 and nuclear factor-κB (NFκB)-dependent manner. Signal 2: Pro IL-1β and IL-18 become activated in this pathway. HCV p7, an ion channel protein, promotes potassium efflux that activates the nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome. Utilizing apoptosis-associated speck-like protein containing a CARD as an adaptor protein, NLRP3 activates caspase-1, which induces the maturation of pro-IL-1β and pro-IL-18 into their active forms. HCV core and NS3 proteins interact with TLR1 or TLR6 and TLR2 to activate NFκB, which results in the production of TNF-α, IL-6, and IL-10. Additionally, HCV particles can also be recognized by TLR7/8, inducing TNF-α production. TLR tolerance is a protection mechanism against uncontrolled inflammation. In HCV infection, it can be abrogated by interferon-γ through NF-κB signaling, leading to the production of high levels of proinflammatory cytokines. HCV: Hepatitis C virus; HCVc: HCV core protein; IL: Interleukin; TLR: Toll-like receptor; TNF-α: Tumor necrosis factor α; DC-SIGN: Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; TLR: Toll-like receptor; MYD88: Myeloid differentiation primary response gene 88; NF-κB: Nuclear factor-κB; NLRP3: Nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3; ASC: Apoptosis-associated speck-like protein containing a CARD; NS3: Nonstructural protein 3; IFN-γ: Interferon-γ.
- Citation: Yang Y, Tu ZK, Liu XK, Zhang P. Mononuclear phagocyte system in hepatitis C virus infection. World J Gastroenterol 2018; 24(44): 4962-4973
- URL: https://www.wjgnet.com/1007-9327/full/v24/i44/4962.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i44.4962