©The Author(s) 2017.
World J Gastroenterol. Feb 7, 2017; 23(5): 817-829
Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.817
Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.817
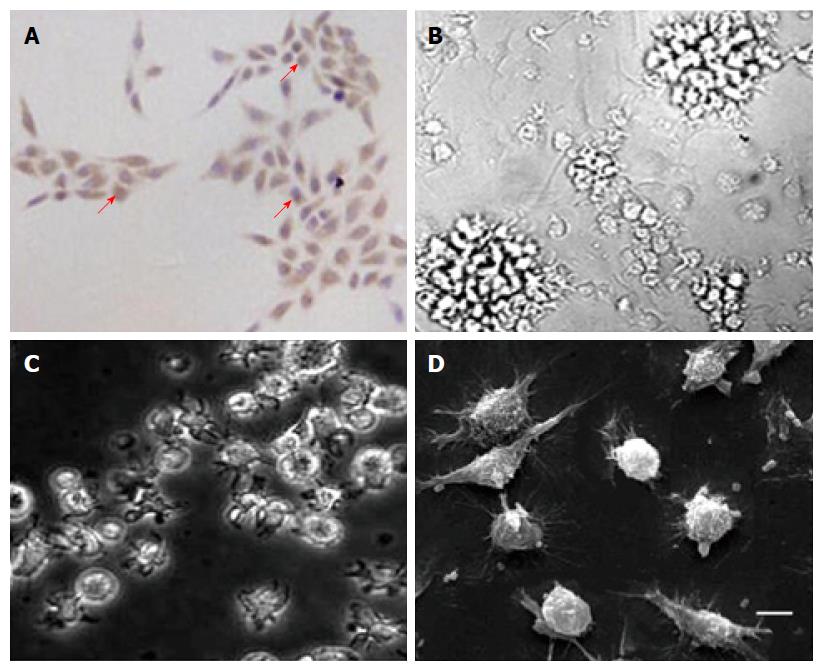
Figure 1 Cells obtained represented typical morphological characteristic of primary tumor cells and mature dendritic cells.
Cells were derived from five pancreatic cancer (PC) patients and with similar shape of target cells. A: Most of the cultured primary tumor cells (over 90%) showed a DcR3-positive expression (magnification × 200); B: After cultured for 7 d, matured dendritic cells (DCs) were transfected without RNA and assembled into non-cohesive colonies, and the ablated cell showed a distinctive villiform process (magnification × 200). DCs transfected with total tumor RNA alone (C) or together with anti-DcR3 mAb mRNA (D) showed similar cell morphology to DCs transfected without RNA (C: magnification × 400; D: scanning electron microscope, bar represents 10 μm).
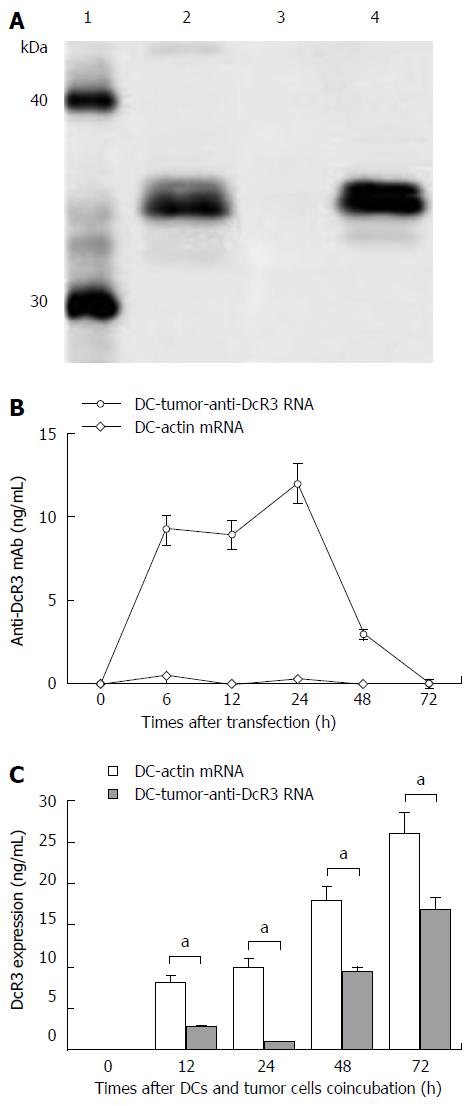
Figure 2 Identification, concentration and function of anti-DcR3 monoclonal antibody secreted by dendritic cells co-transfected with total tumor RNA and anti-DcR3 monoclonal antibody mRNA.
A: Western blotting analysis showed that, similar to commercial anti-DcR3 mAb (lane 2), mAb secreted by dendritic cells (DCs) co-transfected with total tumor RNA and humanized anti-DcR3 H+L mRNA (lane 4) could also react with the recombinant human DcR3 protein (molecular weight of 35 kDa) and generate a band with molecular weight slightly greater than 30 kDa, whereas the supernatant harvested from DC-actin RNA could not bind the DcR3 protein (lane 3); B: The amounts of anti-DcR3 mAb produced by RNA transfected DCs were analyzed using indirect ELISA assay. The mAb secreted by DC-tumor-anti-DcR3 RNA was transient, peaked at 24 h, and then could not be detected after 72 h. However, no anti-DcR3 mAb was found in the supernatant of DC-actin RNA continuously; C: The specific antigen binding effect of anti-DcR3 mAb secreted by RNA transfected DCs was determined by measuring the levels of DcR3 protein in the supernatant of autologous tumor cells (1 × 106 cells) co-cultured with defined DCs (1 × 106 cells). After co-incubation with DC-tumor-anti-DcR3 RNA for 12-72 h, the soluble DcR3 protein level in the supernatant of autologous PC cells was significantly lower than those of tumor cells and the DC-actin RNA co-cultured group (aP < 0.05). Except for those of western blotting and histogram, data represent the means of three experiments, and the histograms are representative of three experiments. Error bars represent SD. mAb: Monoclonal antibody.
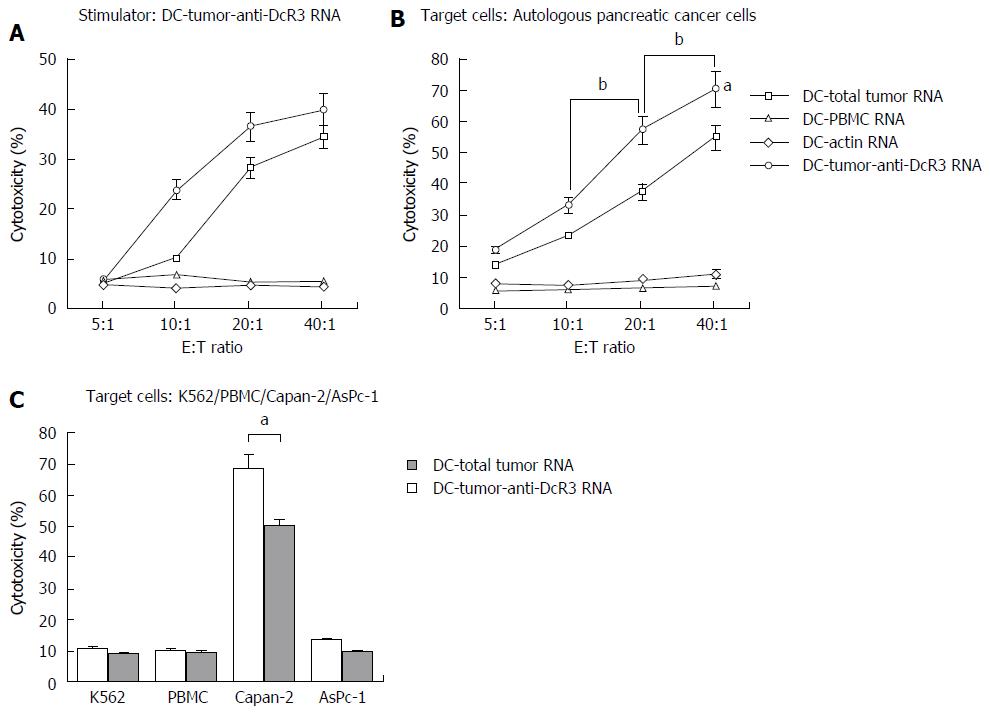
Figure 3 Antigen-specific immune response against pancreatic cancer is enhanced by anti-DcR3 monoclonal antibody secreting dendritic cell.
Dendritic cells (DCs)-total tumor RNA, DC-PBMC RNA, DC-actin RNA and DC-tumor-anti-DcR3 RNA were used to stimulate autologous T cells weekly for two times followed by a cytotoxic T lymphocyte (CTL) assay. Induction of tumor antigen-specific CTLs was measured by using RNA-transfected DCs and tumor targets (primary tumor cells, K562, Capan-2 and AsPC-1 cell line cells). A: Left panel: DC-tumor-anti-DcR3 RNA was used as not only stimulator cells but also target cells, and CTLs stimulated by DC-tumor-anti-DcR3 RNA could recognize and lyse tumor antigen-specific cancer targets (DC-total tumor RNA and DC-tumor-anti-DcR3 RNA). No cross-reactivity was apparent against DCs loaded with normal tissue surrounding PC or actin (DC-PBMC RNA and DC-actin RNA). Compared with CTLs stimulated by DC-total tumor RNA, DC-tumor-anti-DcR3 RNA further enhanced the induction of CTL activity. Right panel: Both DCs co-transfected with total tumor RNA and anti-DcR3 mAb mRNA and DCs transfected with total tumor RNA alone showed an effective and superior ability in recognizing and lysing HLA-A2+ autologous PC cells, whereas T cells activated by DC-PBMC RNA or DC-actin RNA could not (aP < 0.05). Moreover, CTLs induced by DC-tumor-anti-DcR3 RNA could produce a more powerful killing activity toward the tumor cells compared with the DC-total tumor RNA with the E:T ratio increasing from 10:1 to 40:1 (bP < 0.05); B: At the E:T ratio of 40:1, the effector T cells (HLA-A2+) stimulated by DCs transfected with total tumor RNA alone or together with anti-DcR3 mAb mRNA could lyse the Capan-2 cell line cells, which endogenously expressed the HLA-A2 antigen effectively. By contrast, the cells of the AsPC-1 line (HLA-A2-) were not recognized and lysed. DC-tumor-anti-DcR3 RNA showed a more powerful capability in inducing the cytotoxicity of CTLs against HLA-A2-matched tumor cell line (Capan-2) compared with that induced by DC-total tumor RNA alone (aP < 0.05). The experiment was repeated thrice representatively, and the data are shown as mean ± SD. mAb: Monoclonal antibody; DC: Dendritic cell; CTLs: Cytotoxic T lymphocytes.
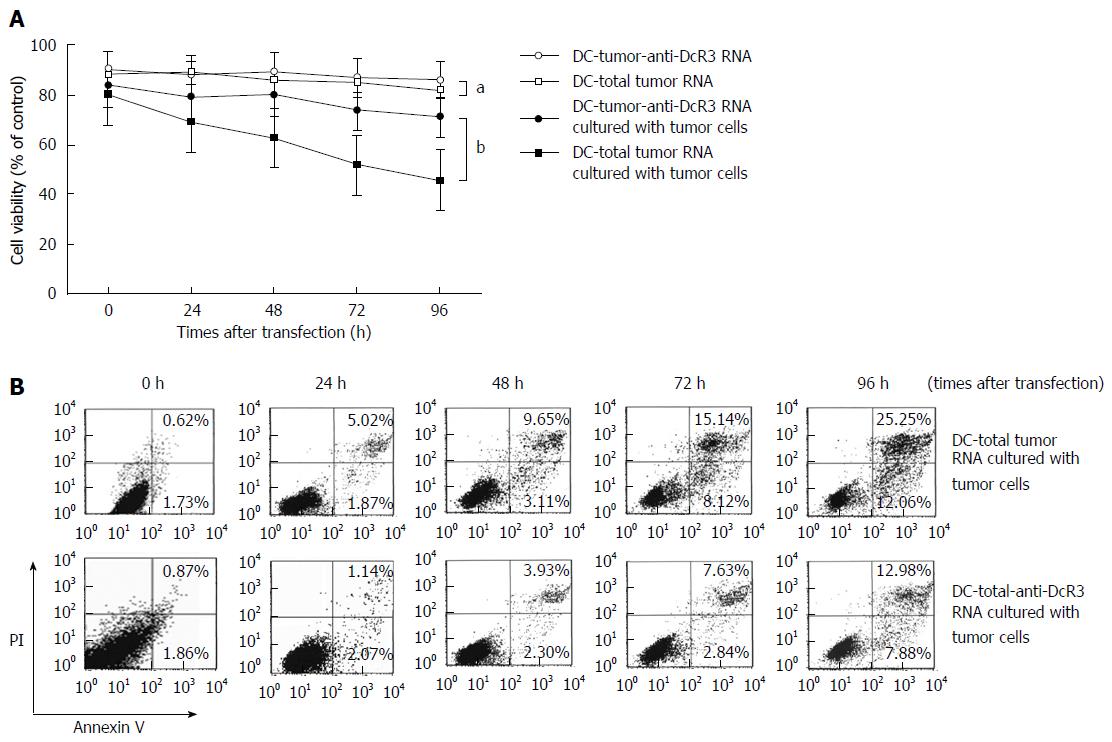
Figure 4 Effects of anti-DcR3 monoclonal antibody secreting dendritic cells on cell viability and apoptosis in tumor RNA-loaded dendritic cells.
A: Dendritic cells (DCs) secreting anti-DcR3 mAb improved the viability of RNA-loaded DCs. The viabilities of DC-total tumor RNA and DC-tumor-anti-DcR3 RNA, cultured with or without autologous tumor cells, were measured using MTT assay after transfection for 0-96 h. The viability of DCs transfected with total tumor RNA alone or together with anti-DcR3 mAb mRNA did not change significantly at the designed time points, with approximately 85% survival throughout (aP > 0.05). Within the same period, the viability of DC-total tumor RNA cultured with tumor cells was evidently lower than that of DC-tumor-anti-DcR3 RNA and decreased in a time-dependent manner (bP < 0.01). Three representative experiments were run, and the data are shown as mean ± SD; B: DC-total tumor RNA and DC-tumor-anti-DcR3 RNA were co-cultured with autologous pancreatic cancer (PC) cells (1 × 106) for 0, 24, 48, 72 and 96 h. Cells stained with annexin V-FITC and propidium iodide were analyzed by flow cytometry. The percentage of annexin V-positive apoptotic cells in DCs transfected with total tumor RNA markedly increased in a time-dependent manner, whereas the apoptotic cells increased slowly in DCs co-transfected with total tumor RNA and anti-DcR3 mAb mRNA. mAb: Monoclonal antibody.
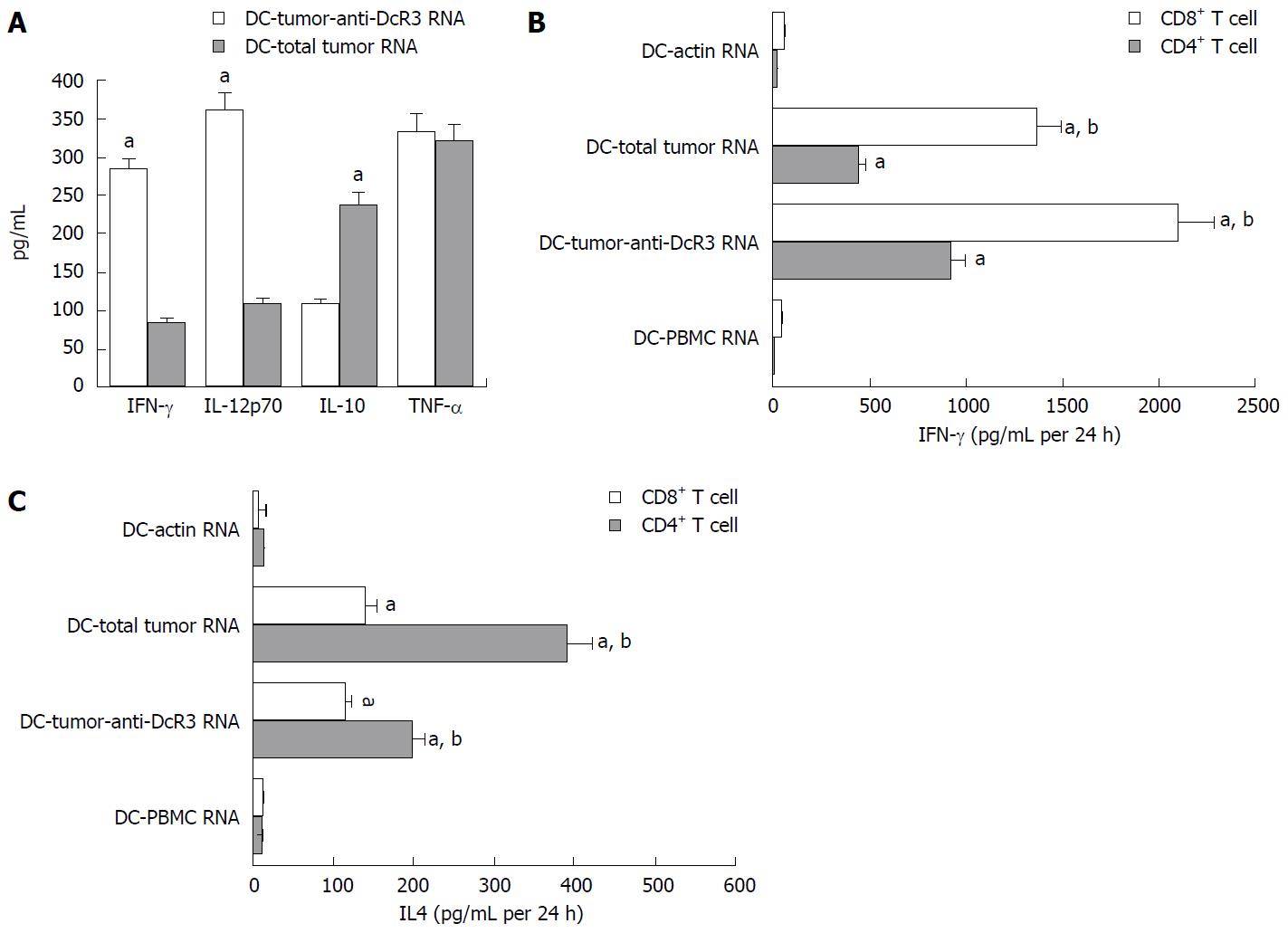
Figure 5 T cell responses induced by anti-DcR3 monoclonal antibody secreting dendritic cells.
The T cells were co-cultured with RNA-dendritic cells (DCs) for 24 h. Then, the supernatants were collected, and the cytokine levels were measured by ELISA assay. A: ELISA test showed the cytokines of IL-12p70 and IFN-γ secreted by T cells pulsed by DC-tumor-anti-DcR3 RNA were higher than those secreted by T cells pulsed by DC-total tumor RNA without significant change of TNF-α. Meanwhile, IL-10 level was lower in DC-tumor-anti-DcR3 RNA than in DC-total tumor RNA (aP < 0.05); B: CD4+ T cells and CD8+ T cells incubated with DCs encoding whole tumor antigens could produce extremely higher IFN-γ levels compared with those incubated with DCs as control or DCs treated with normal tissues (aP < 0.01). Furthermore, the CD4+ T and CD8+ T cells incubated with DCs co-transfected total tumor RNA and anti-DcR3 mAb mRNA produced higher IFN-γ levels than those incubated with DC-total tumor RNA (bP < 0.01); C: The CD4+ T and CD8+ T cells incubated with DCs loaded with whole tumor antigens showed a positive expression of IL-4, whereas a negative or weak expression of IL-4 was observed in DC-actin RNA and DC-PBMC RNA cells (aP < 0.01). Moreover, decreased levels of IL-4 production were detected in CD4+ T cells stimulated by DCs co-transfected with total tumor RNA and anti-DcR3 mAb mRNA compared with DCs transfected with total tumor RNA individually (bP < 0.01). The experiment was repeated three times representatively, and results are shown as mean ± SD. IL: Interleukin; ELISA: Enzyme-linked immunosorbent assay; PBMC: Peripheral blood monocyte cell; mAb: Monoclonal antibody.
- Citation: Chen J, Guo XZ, Li HY, Zhao JJ, Xu WD. Dendritic cells engineered to secrete anti-DcR3 antibody augment cytotoxic T lymphocyte response against pancreatic cancer in vitro. World J Gastroenterol 2017; 23(5): 817-829
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/817.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.817