©The Author(s) 2015.
World J Gastroenterol. Aug 14, 2015; 21(30): 9189-9208
Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9189
Published online Aug 14, 2015. doi: 10.3748/wjg.v21.i30.9189
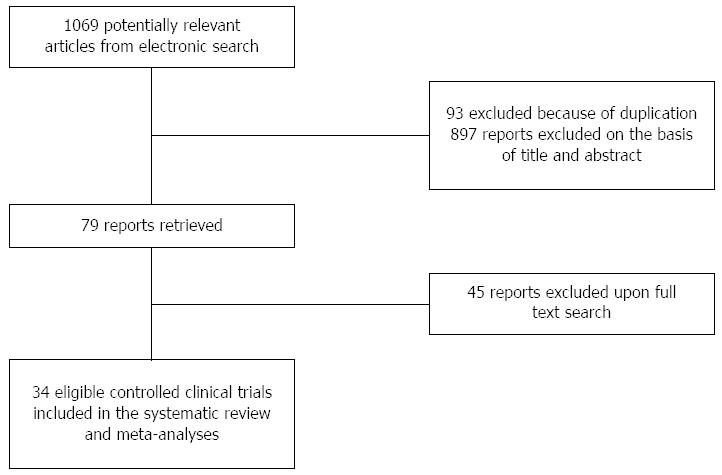
Figure 1 Flow diagram of study selection.
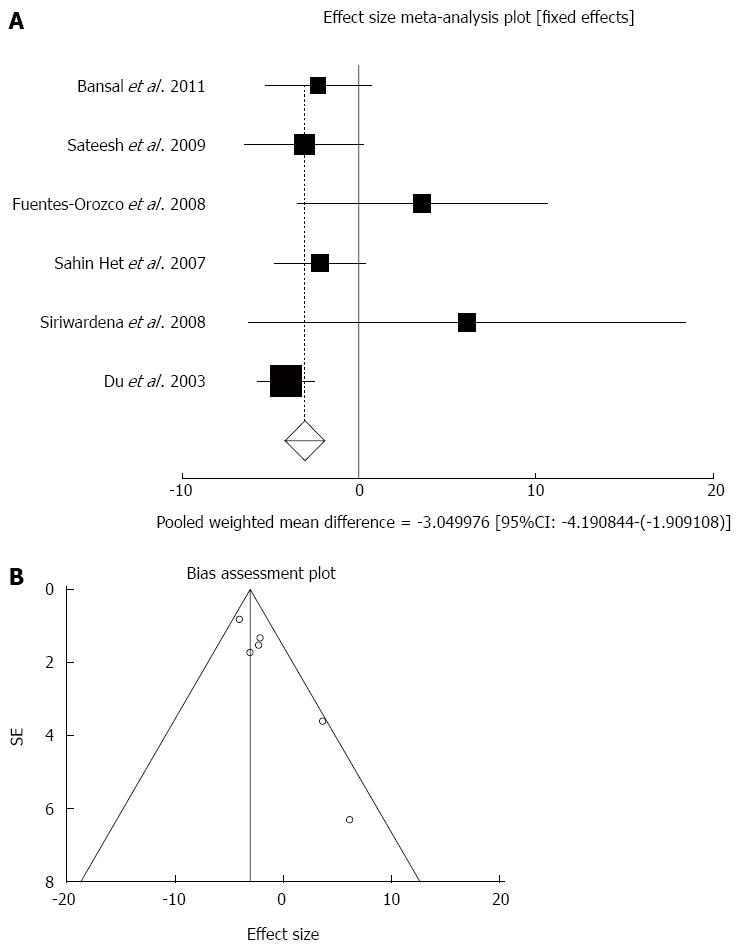
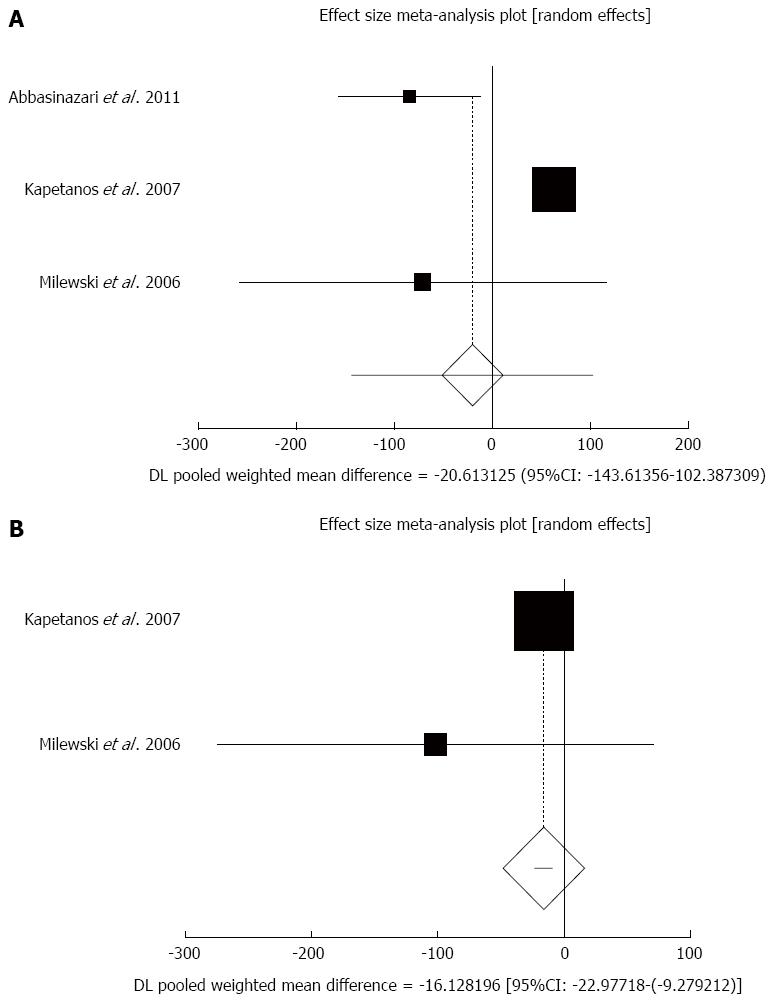
Figure 2 Individual and pooled effect size for standardized mean for the outcome of “rate of hospitalization in acute pancreatitis” in the studies considering antioxidants compared to placebo therapy in 303 patients (A) and publication bias indicators for the outcome of “rate of hospitalization in chronic pancreatitis” in the studies considering antioxidants compared to placebo therapy in 303 patients (B).
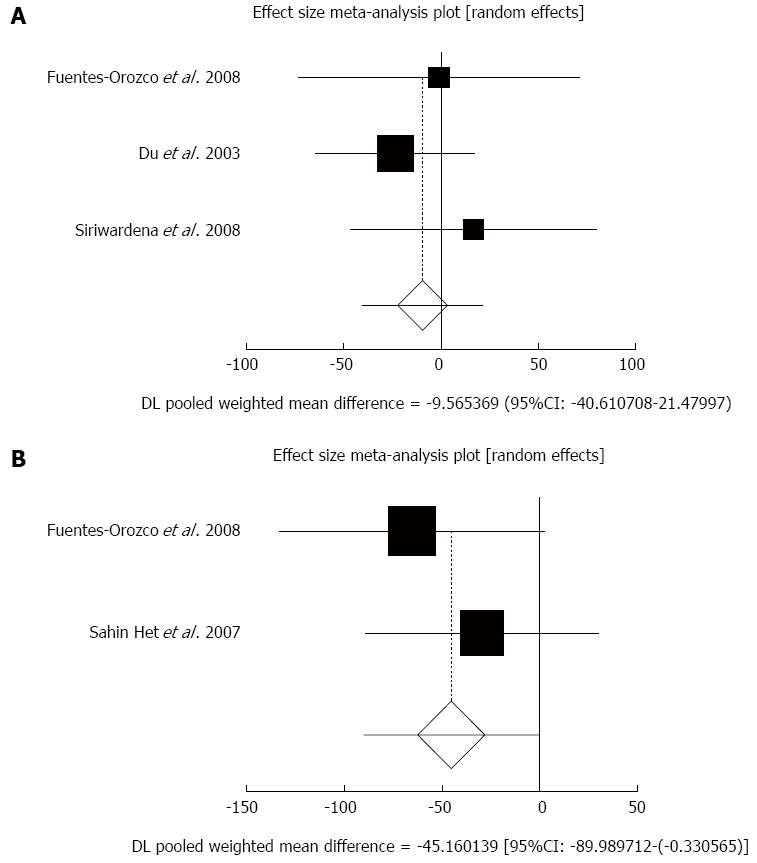
Figure 3 Individual and pooled effect size for standardized mean for the outcome of “serum C reactive protein in acute pancreatitis patients after 5-7 d sampling” in the studies considering antioxidants compared to placebo therapy in 171 patients (A) and individual and pooled effect size for standardized mean for the outcome of “serum C reactive protein in acute pancreatitis patients after 10 d sampling” in the studies considering antioxidants compared to placebo therapy in 84 patients (B).
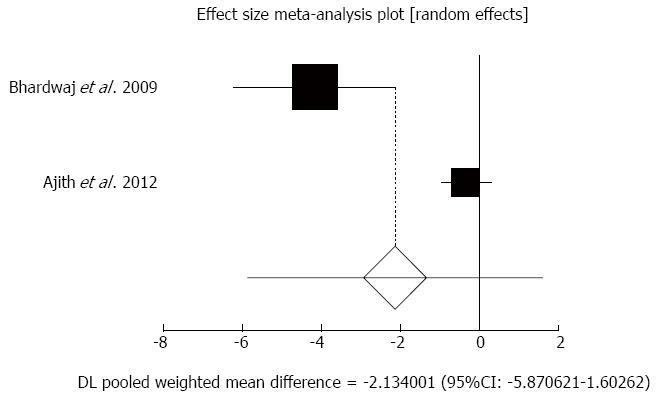
Figure 4 Individual and pooled effect size for standardized mean for the outcome of “pain in chronic pancreatitis patients” in the studies considering antioxidants compared to placebo therapy in 189 patients.
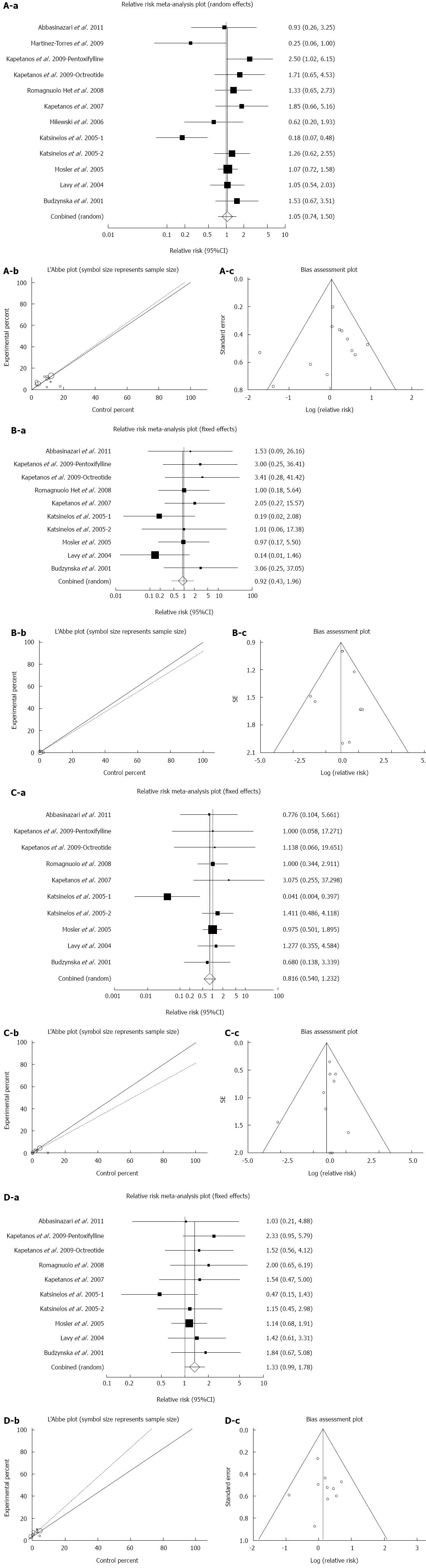
Figure 5 Effect of antioxidants compared with placebo therapy on incidence.
Individual and pooled relative risk (A-a), heterogeneity indicators for (A-b), and publication bias indicators for (A-c) the outcome of “all types of PEP” in the studies considering antioxidants compared to placebo therapy in 1849 patients undergoing ERCP; individual and pooled relative risk (B-a); Heterogeneity indicators (B-b); and publication bias indicators (B-c) for the outcome of “severe PEP” in the studies considering antioxidants compared to placebo therapy in 1709 patients undergoing ERCP; individual and pooled relative risk (C-a); heterogeneity indicators for (C-b); publication bias indicators (C-c) for the outcome of “moderate PEP” in the studies considering antioxidants compared to placebo therapy in 1709 patients undergoing ERCP; individual and pooled relative risk (D-a); heterogeneity indicators (D-b); publication bias indicators (D-c) for the outcome of “mild PEP” in the studies considering antioxidants compared to placebo therapy in 1709 patients undergoing ERCP. PEP: Post-endoscopic retrograde cholangiopancreatography pancreatitis; ERCP: Endoscopic retrograde cholangiopancreatography.
Figure 6 ndividual and pooled effect size for standardized mean for the outcome.
A: Of “serum amylase in patients undergoing ERCP after less than 8 h sampling” in the studies considering antioxidants comparing to Placebo therapy in 500 patients; B: Of “serum amylase of patients undergoing ERCP after less than 24 h sampling” in the studies considering antioxidants comparing to Placebo therapy in 426 patients. ERCP: Endoscopic retrograde cholangiopancreatography.
- Citation: Gooshe M, Abdolghaffari AH, Nikfar S, Mahdaviani P, Abdollahi M. Antioxidant therapy in acute, chronic and post-endoscopic retrograde cholangiopancreatography pancreatitis: An updated systematic review and meta-analysis. World J Gastroenterol 2015; 21(30): 9189-9208
- URL: https://www.wjgnet.com/1007-9327/full/v21/i30/9189.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i30.9189