©The Author(s) 2015.
World J Gastroenterol. Apr 14, 2015; 21(14): 4195-4209
Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4195
Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4195
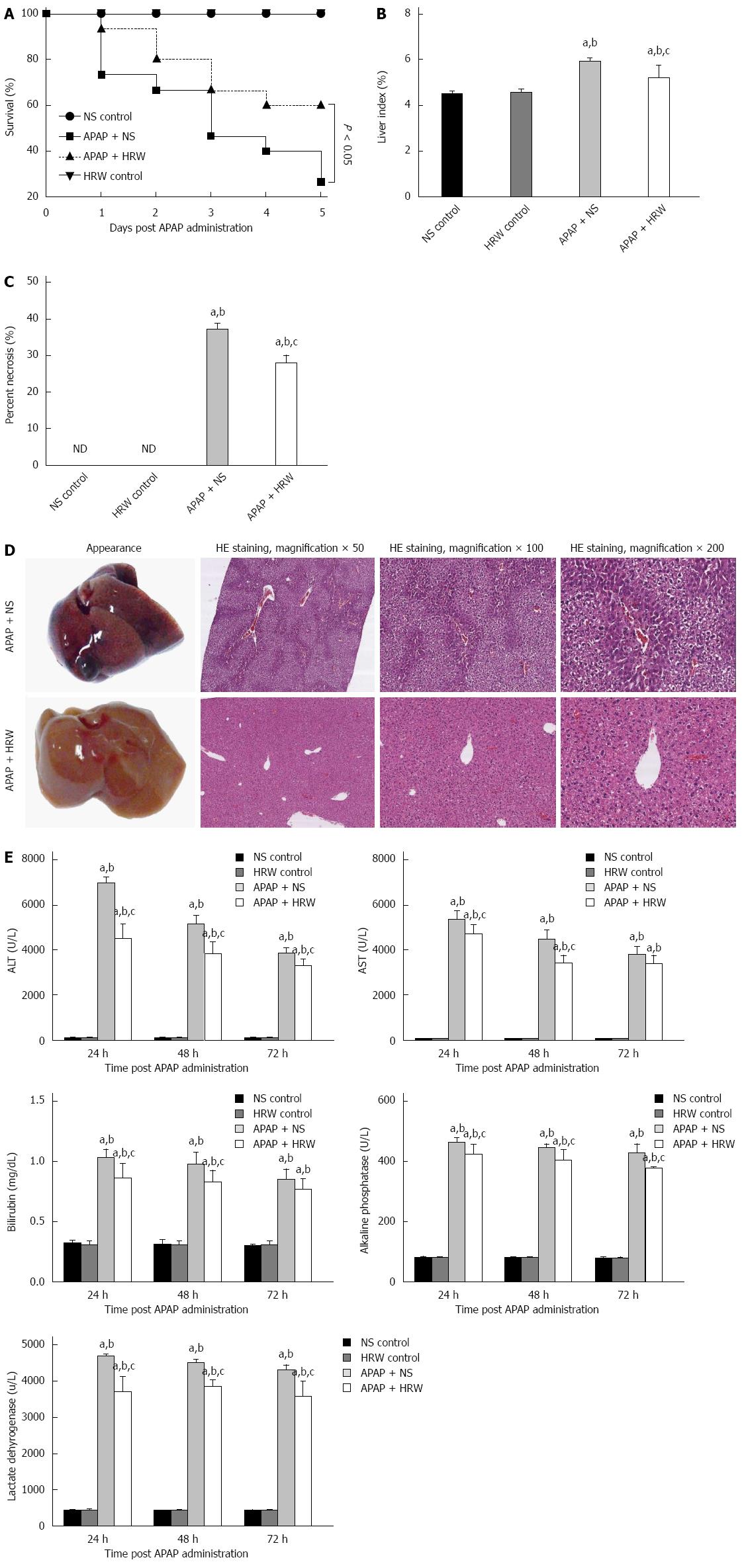
Figure 1 Hydrogen-rich water leads to an obvious enhancement in the survival and decrease in the liver injury after acetaminophen challenge.
A: Kaplan-Meier survival curve for mice in three groups in 5 d after a single, lethal dose of 750 mg/kg acetaminophen (APAP) [normal saline (NS) control or hydrogen-rich water (HRW) control groups, n = 5; APAP + NS or APAP + HRW groups, n = 15]; B: Liver tissues were harvested 3 d after a sub-lethal dose of 500 mg/kg APAP. The liver index was used to evaluate the liver injury and it was decreased by HRW administration (n = 6, mean ± SD, aP < 0.05 vs NS control group, bP < 0.05 vs HRW control group, and cP < 0.05 vs APAP + NS group; C: Percent necrosis of mice in different groups (n = 6, mean ± SD, aP < 0.05 vs NS control group, bP < 0.05 vs HRW control group, and cP < 0.05, vs APAP + NS group); D: The representative appearance of livers and HE staining of murine liver sections. Decreased liver hemorrhaging, necrosis, and acute inflammation were observed in the HRW treated mice; E: After a sub-lethal dose of 500 mg/kg APAP administration, blood samples were harvested by tail cutting at 24 and 48 h and eyeball removal at 72 h. Serum was separated by centrifugation from blood to evaluate the liver function. Total bilirubin, alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) at 24, 48 and 72 h were reduced with HRW treatment (n = 6, mean ± SD, aP < 0.05 vs NS control group; bP < 0.05 vs HRW control group; and cP < 0.05 vs APAP + NS group). ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
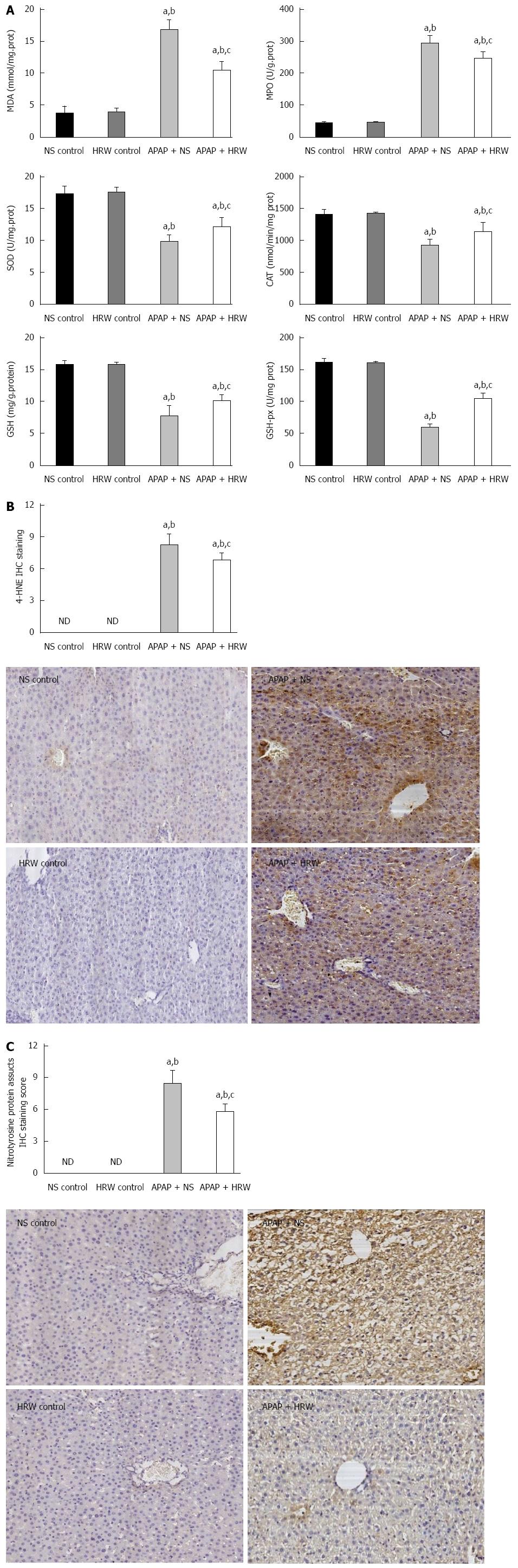
Figure 2 Hydrogen-rich water decreases the oxidative stress and nitrotyrosine formation in the liver after acetaminophen administration.
Three days after a sub-lethal dose of 500 mg/kg acetaminophen (APAP) challenge, blood samples and liver tissues were harvested to evaluate the oxidative stress, inflammation and nitrotyrosine formation. A: Hydrogen-rich water (HRW) protected against APAP-induced elevated malonyldialdehyde (MDA) and myeloperoxidase (MPO) levels, decreased superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) and glutathione peroxidase (GSH-px) activities; B: HRW inhibited the expression of 4-HNE in the liver; C: HRW inhibited the nitrotyrosine (NT) protein adduct formation in the liver (n = 6, mean ± SD, aP < 0.05 vs NS control group; bP < 0.05 vs HRW control group; and cP < 0.05 vs APAP + NS group).
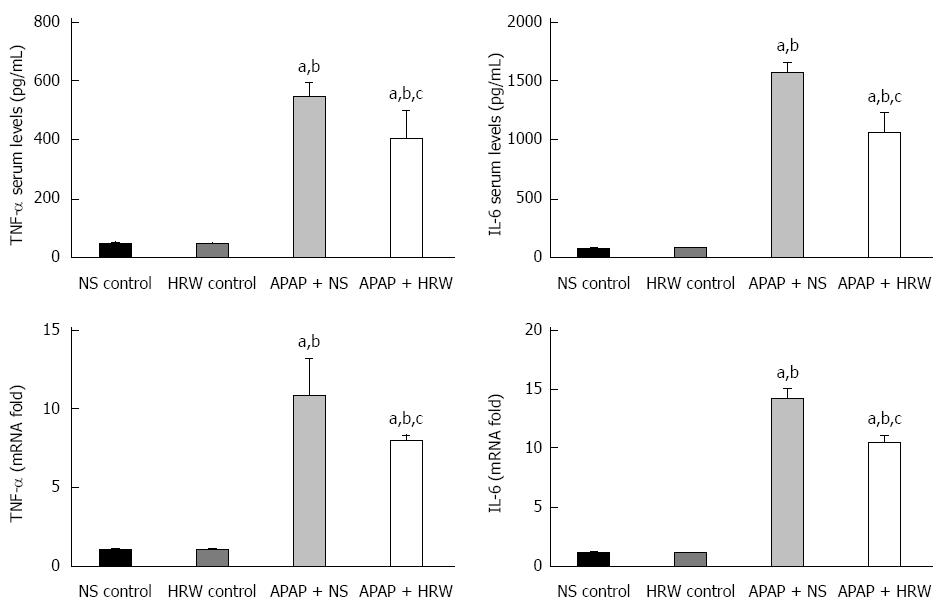
Figure 3 Hydrogen-rich water decreases the inflammation in the liver after acetaminophen administration.
Hydrogen-rich water (HRW) reduced the serum TNF-α and IL-6 concentrations and decreased the transcriptional levels of TNF-α and IL-6 in the liver (n = 6, mean ± SD, aP < 0.05 vs NS control group; bP < 0.05 vs HRW control group; and cP < 0.05 vs APAP + NS group). NS: Normal saline.
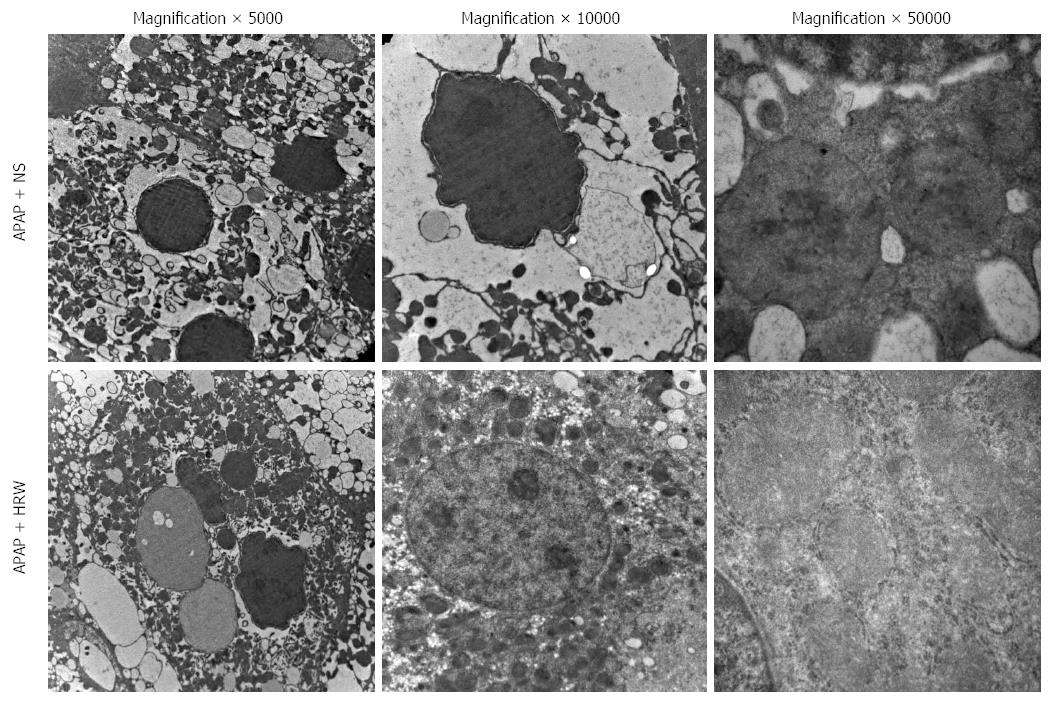
Figure 4 Hydrogen-rich water protects the integrity and stability of organelles after acetaminophen challenge.
Representative electron microscopy of endoplasmic reticulum distension, hyperplasia and dissolution as well as hepatocyte megamitochondria in the acetaminophen (APAP) challenged mice. Hydrogen-rich water (HRW) treatment can alleviate the damage to a relatively normal level. NS: Normal saline.
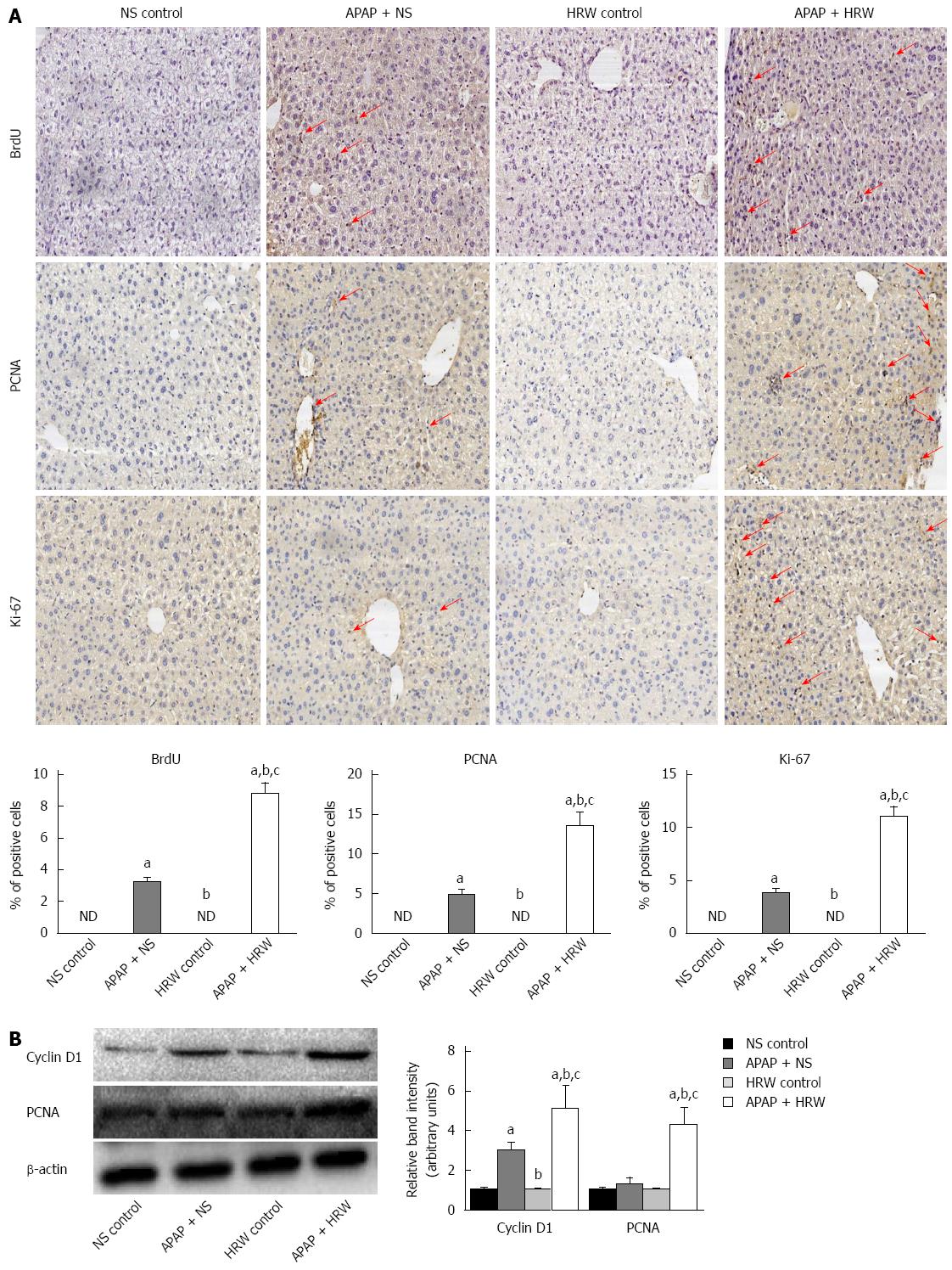
Figure 5 Hydrogen-rich water facilitates hepatocyte proliferation after acetaminophen challenge.
A: Representative immunohistochemistry staining of BrdU, Ki67 and PCNA in the saline plus acetaminophen (APAP) as well as hydrogen-rich water (HRW) plus APAP treatment mice. The quantification of hepatocyte proliferation was assessed by calculating the positive cells observed by microscopy (magnification × 200); B: Western blot analysis for the protein content of cyclin D1 and PCNA proteins in response to APAP and HRW treatment in the liver. β-actin was used as an internal control (n = 6, mean ± SD, aP < 0.05 vs NS control group, bP < 0.05 vs APAP+NS group, and cP < 0.05 vs HRW control group). NS: Normal saline.
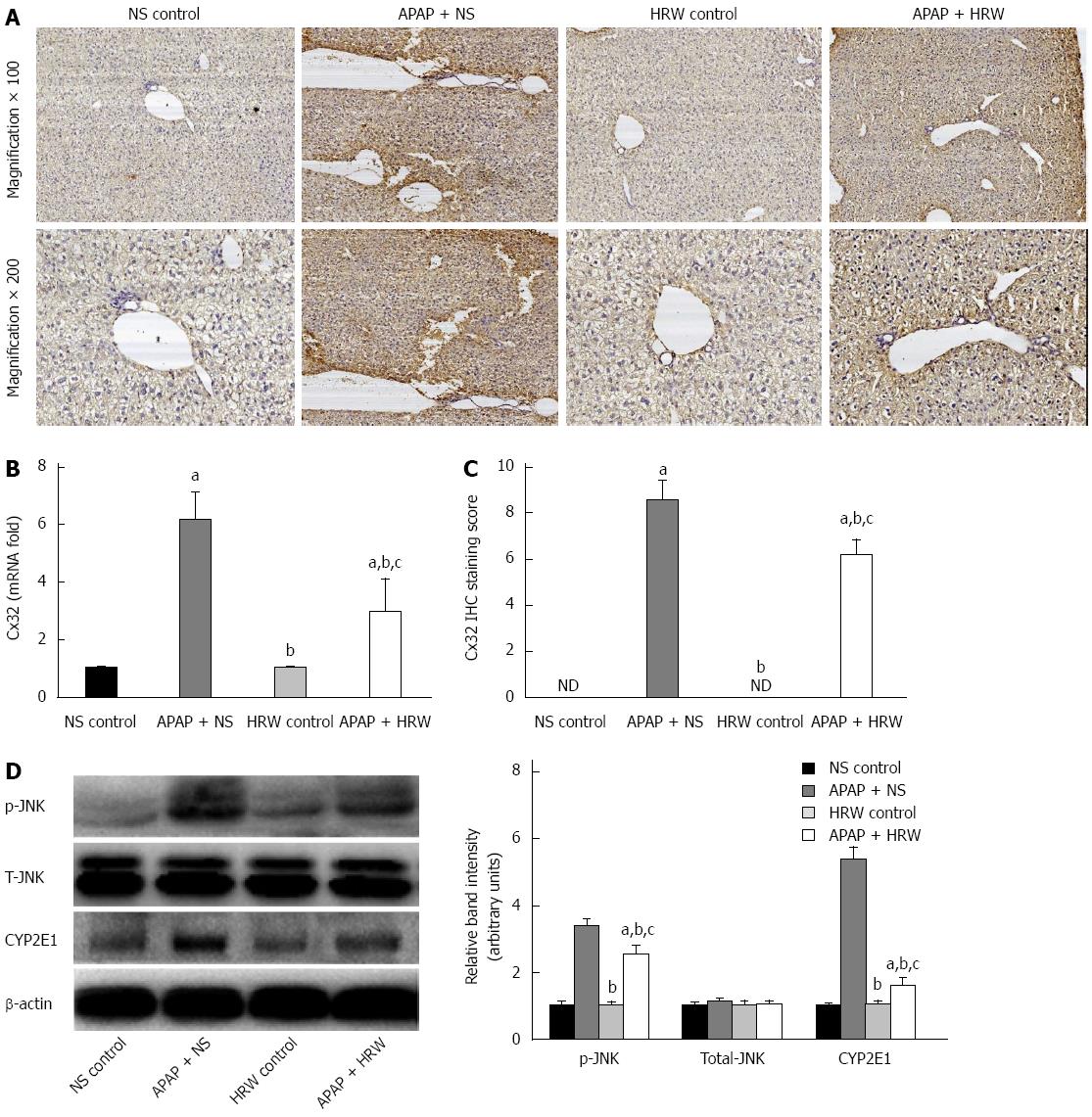
Figure 6 Hydrogen-rich water inhibited connexin 32 expression in the liver tissue.
A: Representative photographs of connexin 32 (Cx32) expression in the liver. The acetaminophen (APAP)-challenged mice had more Cx32 immunopositive staining, as indicated by the brown color, while treatment with hydrogen-rich water (HRW) can significantly reduce Cx32 immunostaining; B: The Cx32 IHC staining score indicated that hydrogen therapy could significantly lower the staining score and reduce its activation and expression; C: The relative Cx32 mRNA levels in the three groups; D: Western blot analysis for the protein content of JNK and cytochrome P4502E (CYP2E1) proteins in response to APAP and HRW treatment in the liver. β-actin was used as an internal control (n = 6, mean ± SD, aP < 0.05 vs NS control group; bP < 0.05 vs APAP + NS group, and cP < 0.05 vs HRW control group). NS: Normal saline.
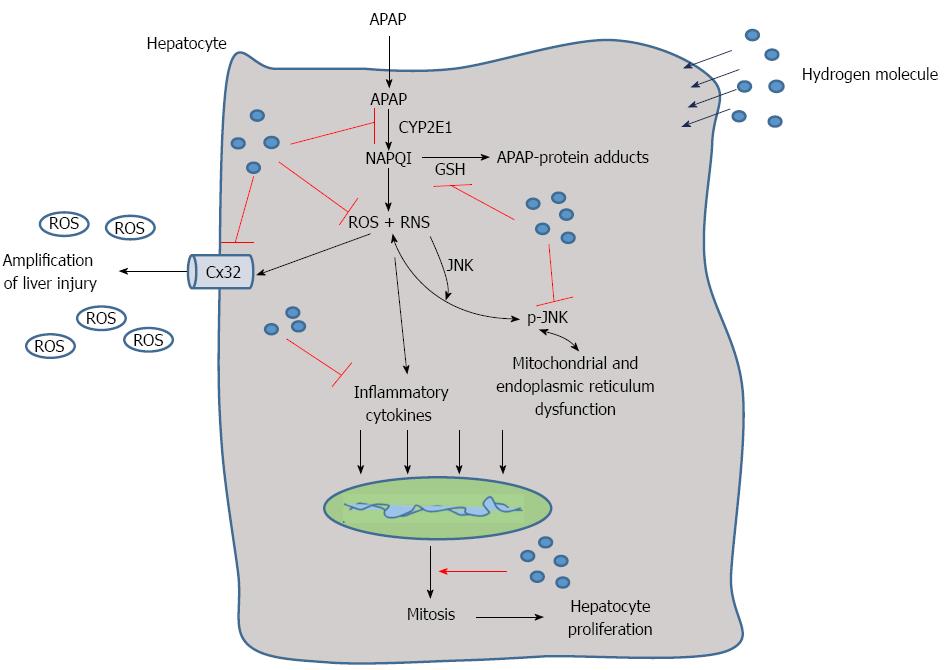
Figure 7 Schematic representation of the proposed effect of the hydrogen molecule in acetaminophen-induced hepatic injury.
After acetaminophen (APAP) administration into the hepatocytes, APAP is first metabolized through the cytochrome P4502E (CYP2E1) and generates N-acetyl-p-benzoquinone imine (NAPQI), which binds with glutathione (GSH) and increases reactive oxygen species (ROS) and RNS formation as well as the level of inflammatory cytokines. It can also cause JNK phosphorylation that damages the mitochondria and endoplasmic reticulum. Meanwhile, connexin 32 (Cx32) can be induced with oxidative stress, which can promote the amplification of liver injury. The hepatic self-repair function is also activated after APAP administration, which can promote the cell mitosis to facilitate liver regeneration. In the present study, we found that hydrogen can reduce APAP hepatotoxicity by inhibiting APAP metabolism, ROS and RNS formation, Cx32 expression and promote hepatocyte proliferation.
- Citation: Zhang JY, Song SD, Pang Q, Zhang RY, Wan Y, Yuan DW, Wu QF, Liu C. Hydrogen-rich water protects against acetaminophen-induced hepatotoxicity in mice. World J Gastroenterol 2015; 21(14): 4195-4209
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4195.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4195