©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Oct 7, 2014; 20(37): 13466-13476
Published online Oct 7, 2014. doi: 10.3748/wjg.v20.i37.13466
Published online Oct 7, 2014. doi: 10.3748/wjg.v20.i37.13466
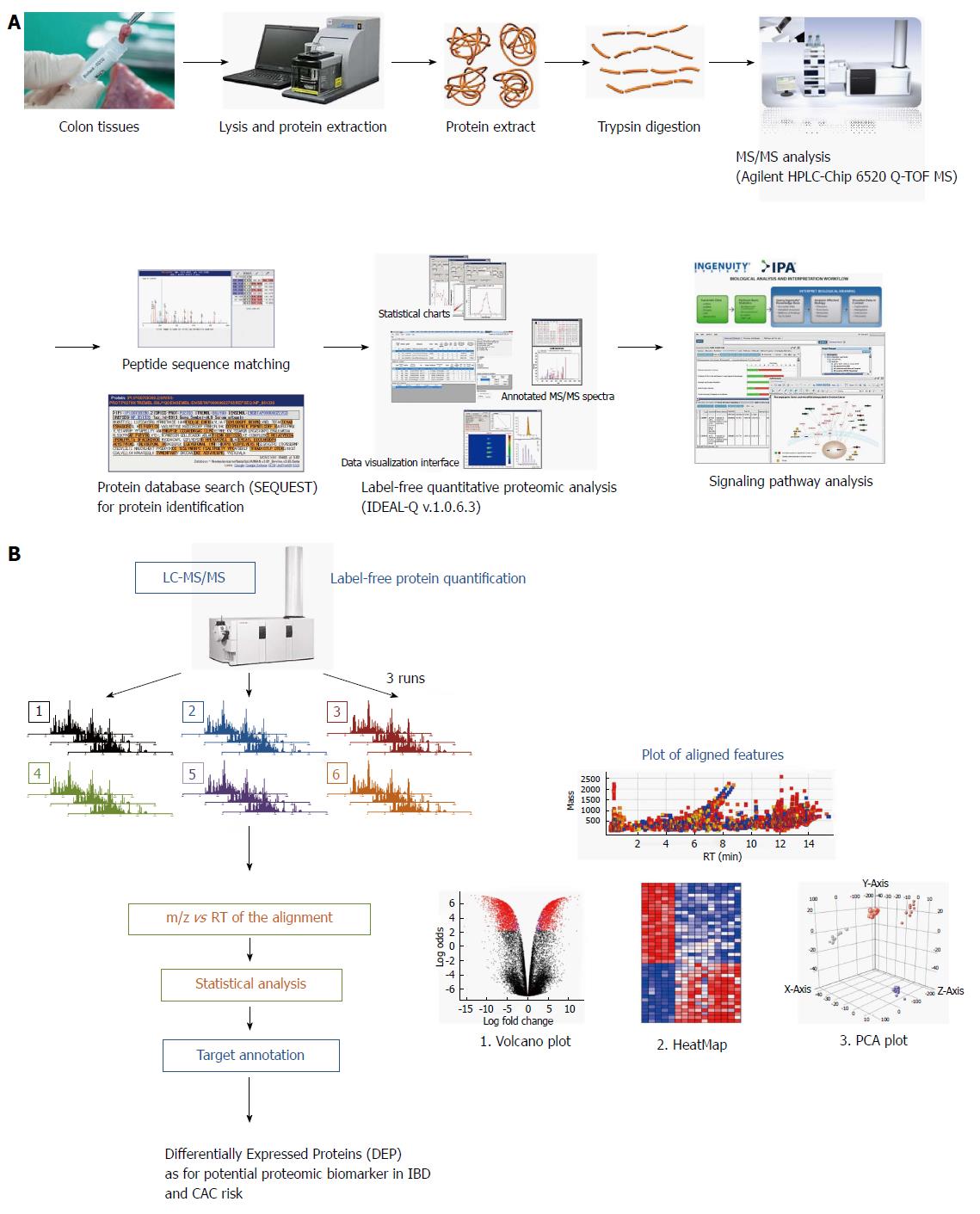
Figure 1 Schematic presentation showing proteome analysis to discover potential biomarkers and label-free quantification analysis in inflammatory bowel disease.
A: Applying the label-free quantification method to discover proteomic biomarkers in patients with different types and different stage of inflammatory bowel disease (IBD). Comparative analysis was done in eight patients with ulcerative colitis (UC), eight patients with Crohn’s disease (CD) and eight patients with irritable bowel syndrome (IBS). Biopsied colon tissues were obtained during colonoscopy after written consent, and stored in a deep freeze until assayed. Using Agilent HPLC-Chip 6520 Q-time-of-flight mass spectrometry (TOF-MS) and label-free quantitative proteome analysis (IDEAL-Q v1.0.6.3), significant signal pathway analysis was done. In the current review, the analysis done according to the degree of intestinal inflammation, type of IBD, and extent of inflammation from 24 patients, eight from non-IBD normal patients; i.e., IBS patients, eight from patients with UC, and from patients with CD; B: Label-free protein quantification scheme for potential biomarker for colitis-associated cancer (CAC) risk in 16 patients with IBD.
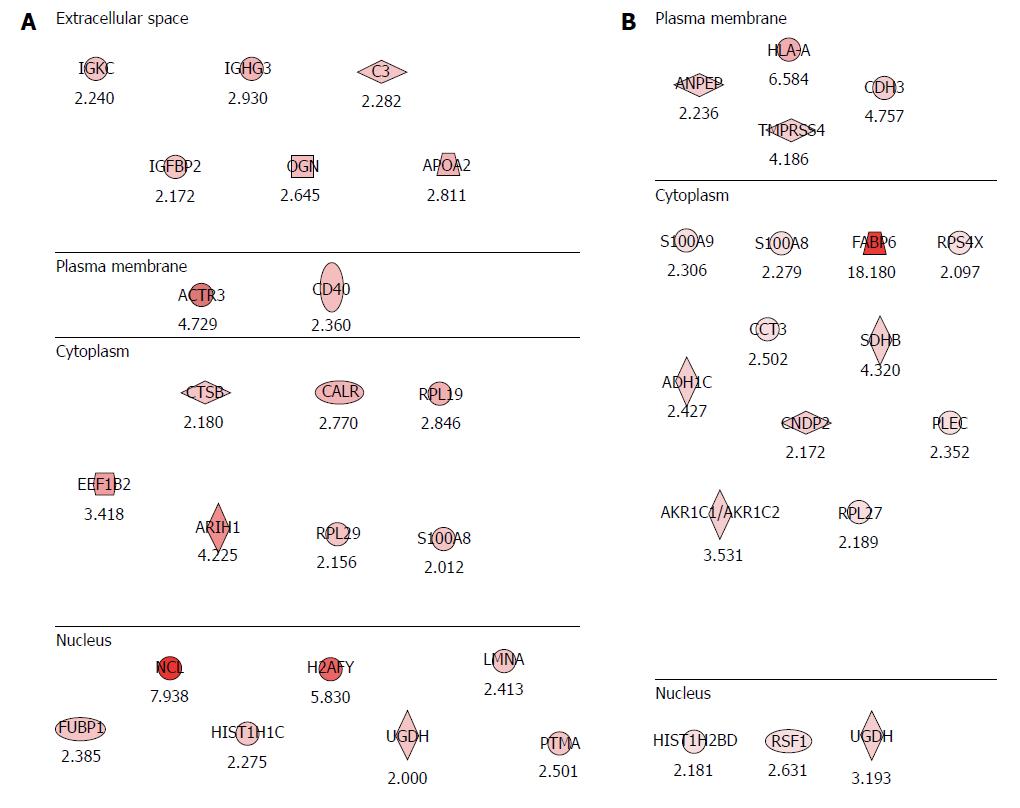
Figure 2 Potential proteomic markers signifying colitis-associated cancer risks in inflammatory bowel disease.
A: Proteomic markers for colitis-associated cancer (CAC) risk in patients with ulcerative colitis (UC). The analysis was performed according to the degree of intestinal inflammation, type of inflammatory bowel disease and extent of inflammation. Compared with the analysis of CAC, 22 significant potential biomarkers of CAC risk were obtained from patients as p with UC, six biomarkers existing in the extracellular space, two biomarkers at the plasma membrane, seven are from the cytoplasm, and seven are nuclear proteins; B: Proteomic markers for CAC risk in patients with Crohn’s disease (CD). Eighteen potential biomarkers for the risk of CAC were identified in patients with CD. Four were from the plasma membrane, 11 from the cytoplasm, and three from the nucleus.
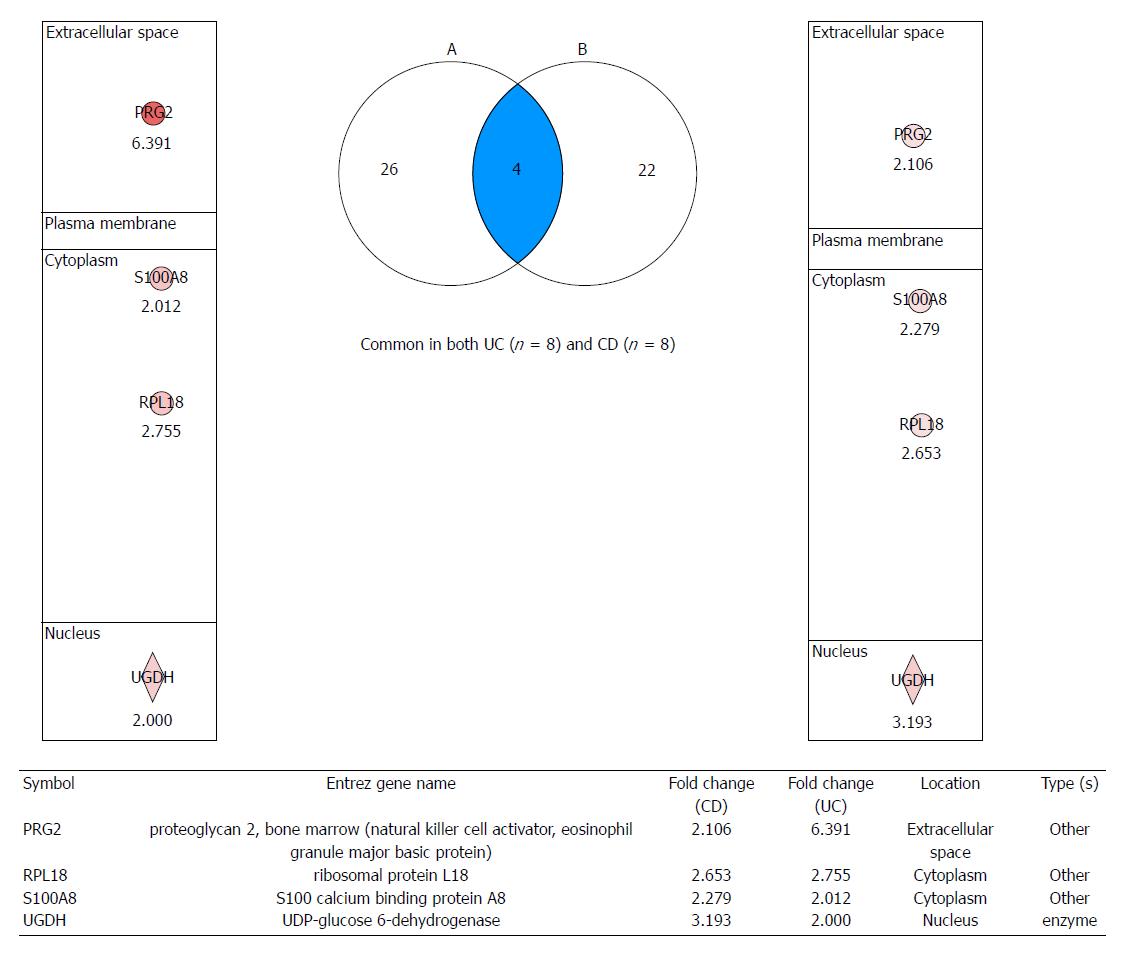
Figure 3 Proteomic markers for colitis-associated cancer risk in patients with both ulcerative colitis and Crohn’s disease.
After analyzing signaling pathways from label-free quantitative analysis, four important proteome biomarkers were identified: proteoglycan 2 (PRG2), S100 calcium binding protein A8 (S100A8), ribosomal protein L18 (RPL18), and UDP-glucose dehydrogenase (UGDH), all of which showed fold changes. Validation is ongoing to investigate these biomarkers for predicting colitis-associated cancer risk in patients with inflammatory bowel disease. UC: Ulcerative colitis; CD: Crohn’s disease.
- Citation: Park JM, Han NY, Han YM, Chung MK, Lee HK, Ko KH, Kim EH, Hahm KB. Predictive proteomic biomarkers for inflammatory bowel disease-associated cancer: Where are we now in the era of the next generation proteomics? World J Gastroenterol 2014; 20(37): 13466-13476
- URL: https://www.wjgnet.com/1007-9327/full/v20/i37/13466.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i37.13466