Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 28, 2014; 20(16): 4467-4482
Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4467
Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4467
Figure 1 Andrzej S Tarnawski, MD, PhD, DSc (Med), Professor of Medicine, University of California, Irvine; and VA Long Beach Health Care System, 5901 E.
Seventh Str., Long Beach, CA 90822, United States; Honorary Professor, Chinese University of Hong Kong; Editor-in-Chief, World Journal of Gastroenterology.
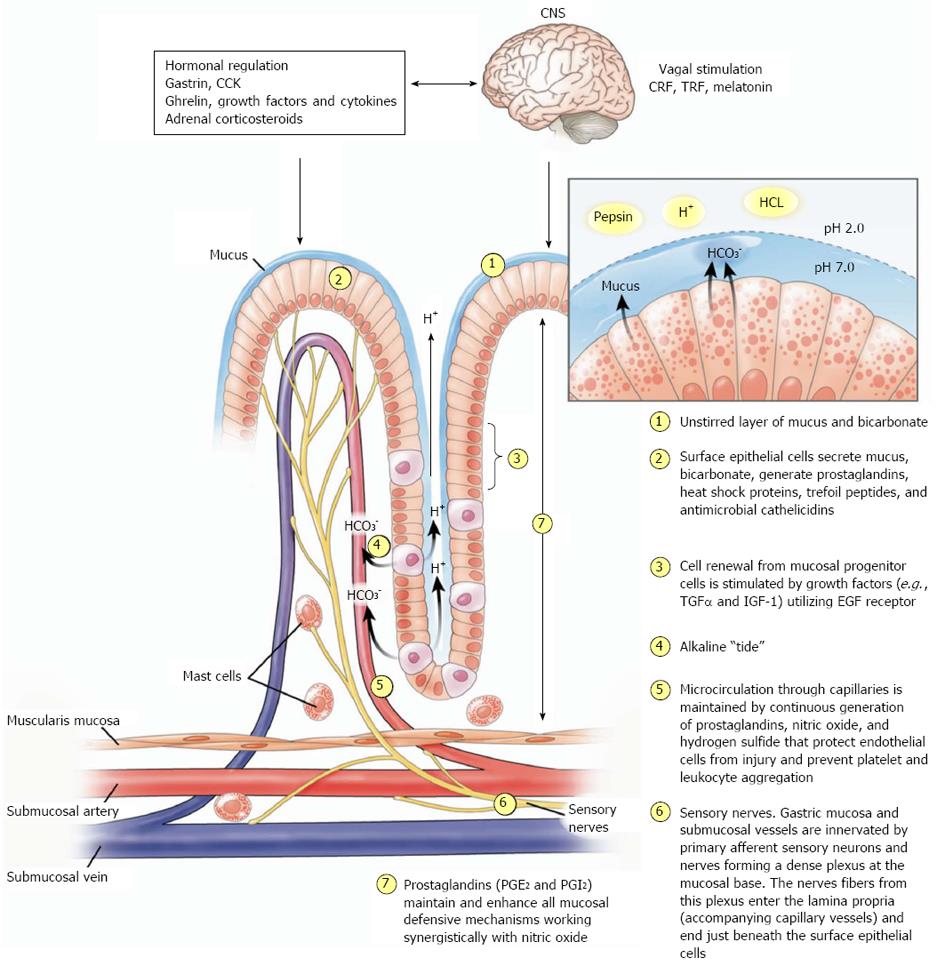
Figure 2 Gastric mucosal defense.
Schematic representation of gastric mucosal defense mechanisms. Reproduced with permission from Laine, Takeuchi and Tarnawski[4]. (1) “Unstirred” layer of mucus/bicarbonate/phospholipids above surface epithelial cells constitutes the first line of defense. It maintains a pH of approximation 7.0 (close to the physiological cell pH) at the surface epithelial cells, while pH in the lumen is about 1.0-3.0; (2) the surface epithelial cells secrete mucus, bicarbonate and synthesize prostaglandins and heat shock proteins; (3) mucosal cell renewal from mucosal progenitor cells is driven by growth factors (transforming growth factor α and insulin like growth factor-1α) utilizing the epidermal growth factor receptors). Expression of survivin in epithelial progenitor cells prevents apoptosis and is the key for “immortality” of these cells under normal conditions; (4) “Alkaline tide”-parietal cells secreting HCl into the gastric gland lumen concurrently secrete bicarbonate into the lumen of adjacent capillary blood vessels. Bicarbonate is transported to the surface and contributes to the first line of defense; (5) mucosal microcirculation through the capillary microvessels is essential for delivery of oxygen and nutrients. Endothelial cells of microvessels generate prostaglandins, mainly PGI2 (prostacyclin) and nitric oxide, which exert vascular and mucosal protective actions; (6) sensory nerve stimulation by H+-ion or other irritants causes release of neurotransmitters such as calcitonin gene related peptide (CGRP) and substance P in nerve terminals, which induce vasodilatation and enhance mucosal blood flow; and (7) continuous generation of prostaglandin E2 (PGE2) and prostacyclin (PGI2) by the gastric mucosal cells is crucial for the maintenance of mucosal integrity. Almost all of the above (1-6) mucosal defense mechanisms are stimulated or facilitated by endogenous or exogenous prostaglandins. CRF: Corticotrophin-releasing factor; TRF: Thyrotropin-releasing factor; CCK: Cholecystokinin.
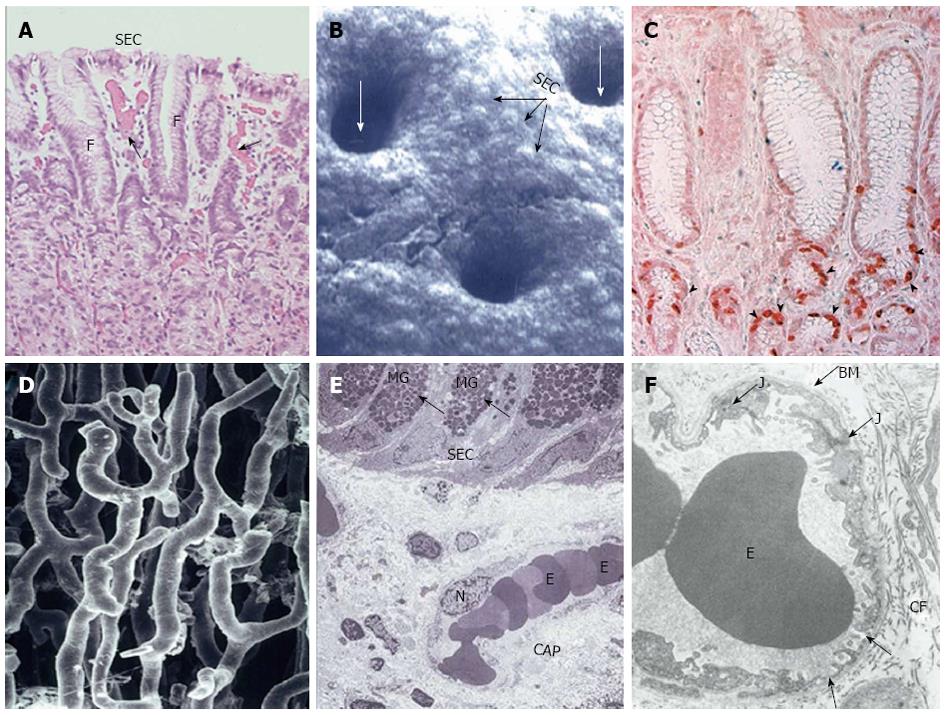
Figure 3 Structural components of gastric mucosal defense: surface epithelial cells, progenitor cells and blood microvessels.
Reproduced with permission from Laine, Takeuchi and Tarnawski[4]. A: Histology of upper part of human gastric mucosa visualizing surface epithelial cells (SEC), foveoli (F), and upper gland area. (Hand E staining; original magnification, × 50). Blood microvessels with erythrocytes in the lumen are present in the lamina propria (arrows); B: Scanning electromicrograph of human gastric mucosal luminal surface. The unstirred mucus gel layer is not seen because of dissolution during fixation. Individual SEC are clearly visible as are lumina of the gastric pits (white arrows). Reproduced with permission from Tarnawski et al[7]; C: Immunostaining of human gastric mucosa with survivin (anti-apoptosis protein) antibody. Survivin is strongly expressed (brown-red staining) in the epithelial progenitor cells located in the foveolar/neck area (arrowheads). Reproduced with permission from Tarnawski et al[1]; D: Vascular cast study of capillary blood vessels in the gastric mucosa using Mercox resin. The remaining components of the mucosa were dissolved with concentrated NaOH. Reproduced with permission from Ichikawa, Tarnawski et al[8]; E: Transmission electron micrograph of normal human gastric mucosa. SEC contain dark mucus granules (MG, arrows). Below the surface epithelial cells, a capillary blood vessel (CAP) with erythrocytes (E) in the lumen is present in the lamina propria. N, nucleus of endothelial cell lining capillary vessel (original magnification, × 2000). Reproduced with permission from Tarnawski et al[9]; F: Transmission electron micrograph of a portion of human gastric capillary blood vessel. The structure of the capillary wall and endothelial cell cytoplasm is normal with a characteristic fenestration (arrows) allowing transport. BM: Basement membrane; E: Erythrocytes in the capillary lumen; J: Junction between two neighboring endothelial cells; CF: Collagen fibers. Original magnification, × 17400. Reproduced with permission from Tarnawski et al[9].
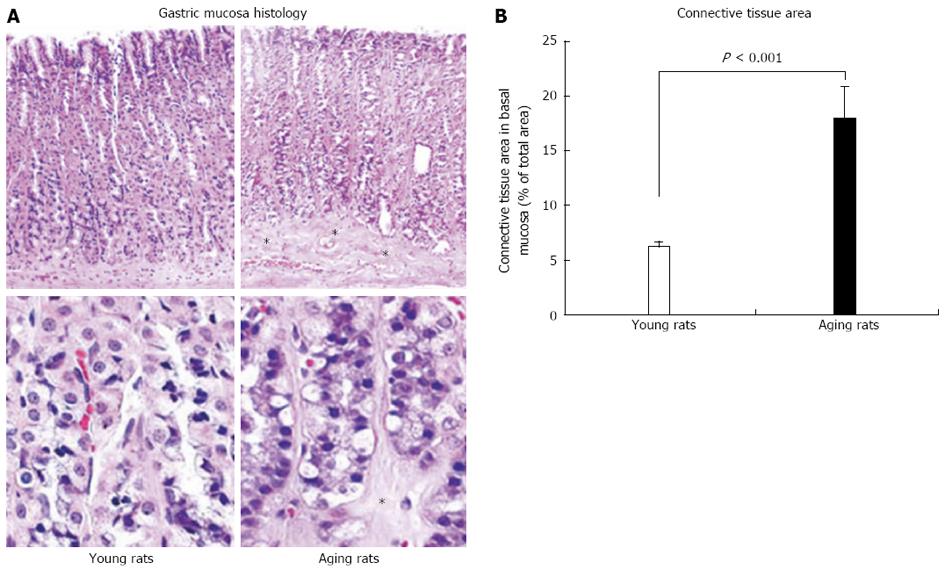
Figure 4 Photomicrographs of gastric mucosa in young and aging rats.
In gastric mucosa of aging rats there is partial atrophy of gastric glands in the basal mucosa and their replacement with connective tissue (*). A: Hematoxylin and eosin staining at low magnification (x 100) is shown in the upper panels and higher magnification (x 500) is shown in the lower panels; B: Quantification of connective tissue in the lower one third of the gastric mucosa shows a significant increase in connective tissue replacing glandular cells in aging rats. Quantification of the number of inflammatory cells in gastric mucosa shows no inflammation (only a minimal number of inflammatory cells) and no difference between young and aging rats indicating that atrophic changes are not accompanied by an inflammation. Reproduced with permission from Tarnawski et al[1].
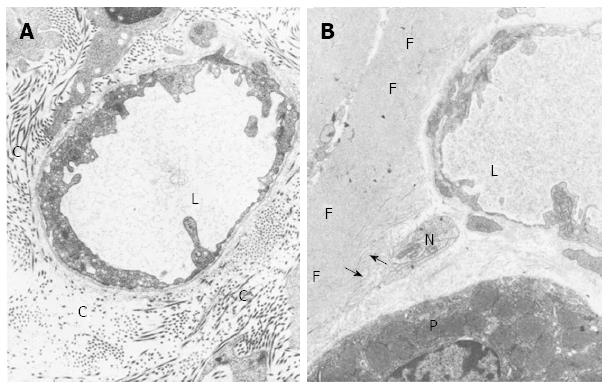
Figure 5 Transmission electron microscopy.
A: Transmission electron micrograph of perivascular connective tissue from a 3-month-old control rat from the basal portion of the oxyntic mucosa. The connective tissue shows numerous collagen fibers (C); L, Microvessel lumen. Magnification x 19000; B: Transmission electron micrograph of perivascular connective tissue from an old rat. In the basal portion of the oxyntic mucosa, collagen fibers are mostly absent and replaced by rudimentary collagen fibers (arrows) and deposits of amorphous fibrillar material (F). P: Parietal cells; L: Blood microvessel lumen; N: Nerve bundle. Magnification x 19000. Reproduced with permission from Hollander, Tarnawski et al[2].
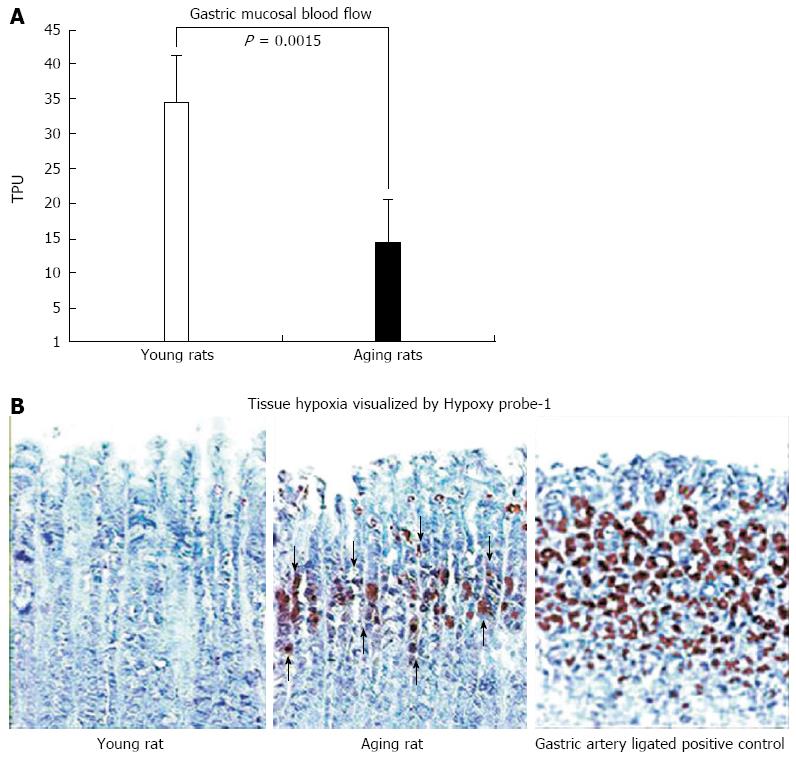
Figure 6 Gastric mucosal blood flow measured with BLF21 Laser-Doppler flow meter and mucosal hypoxia visualized by Hypoxy probe-1.
A: In gastric mucosa of aging rats at baseline, mucosal blood flow, expressed in perfusion units, is significantly reduced by approximation 60% (vs young rats; P = 0.0015). Such a dramatic reduction in blood flow likely leads to chronic hypoxia; B: Photomicrographs of rat gastric mucosa. Gastric mucosal hypoxia is visualized by immunohistochemical staining utilizing the small molecular marker, pimonidazole HCl (Hypoxy probe-1), which binds selectively to oxygen starved cells[30]. In young rats, Hypoxy probe-1 staining is negative in both connective tissue and epithelial cells of the gastric mucosa demonstrating the absence of hypoxia. In aging rats, positive staining is strongly expressed (brown staining) in the upper and mid-mucosa, mainly in the progenitor and parietal cell zone (arrows), reflecting severe hypoxia in these cells. As a positive control we used gastric mucosa of young rats that had all major gastric arteries ligated for 1 h. A strong accumulation Hypoxy probe-1 is present in the majority of epithelial cells (brown staining) reflecting profound cell hypoxia. Reproduced with permission from Tarnawski et al[1].
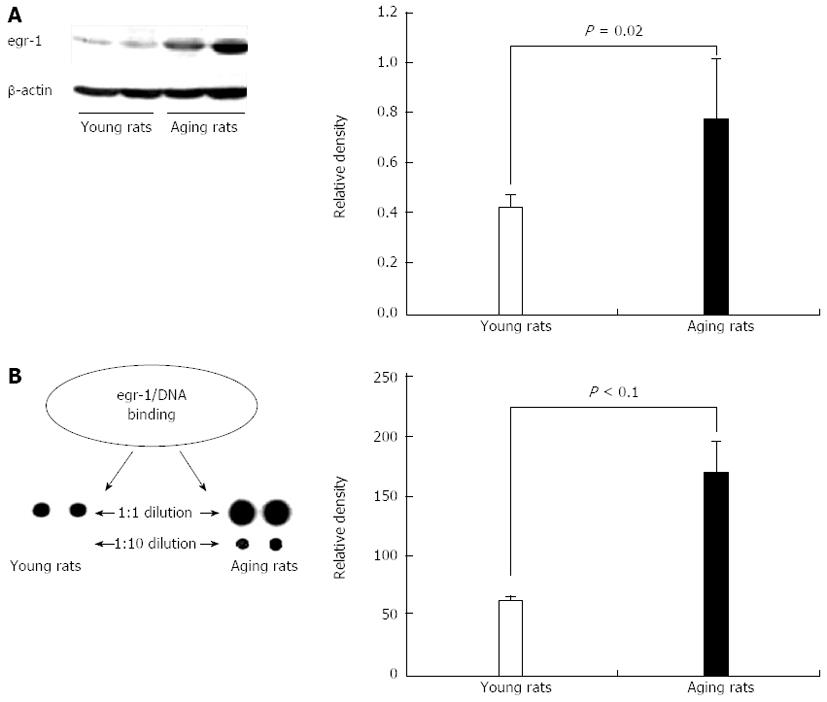
Figure 7 Increased expression of early growth response-1 and increased early growth response-1 transcriptional activity in gastric mucosa of aging vs young rats.
A: Representative Western blotting demonstrate increased early growth response-1 (egr-1) protein expression in gastric mucosa of aging (vs young) rats; B: Assessment of egr-1 transcriptional activity in gastric mucosa of young and aging rats was performed using the TranSignal™ TF-TF Interaction Array (Panomics, Redwood City, CA). The egr-1 cis-element is spotted in duplicate: in the first row DNA was spotted without dilution; in the second row DNA was diluted ten times (1:10). In gastric mucosa of aging rats there is a significant, 2.7-fold increase (vs that of young rats; P < 0.02) in binding of egr-1 protein to its GC-rich cis elements that are highly expressed in the PTEN gene promoter. Reproduced with permission from Tarnawski et al[1].
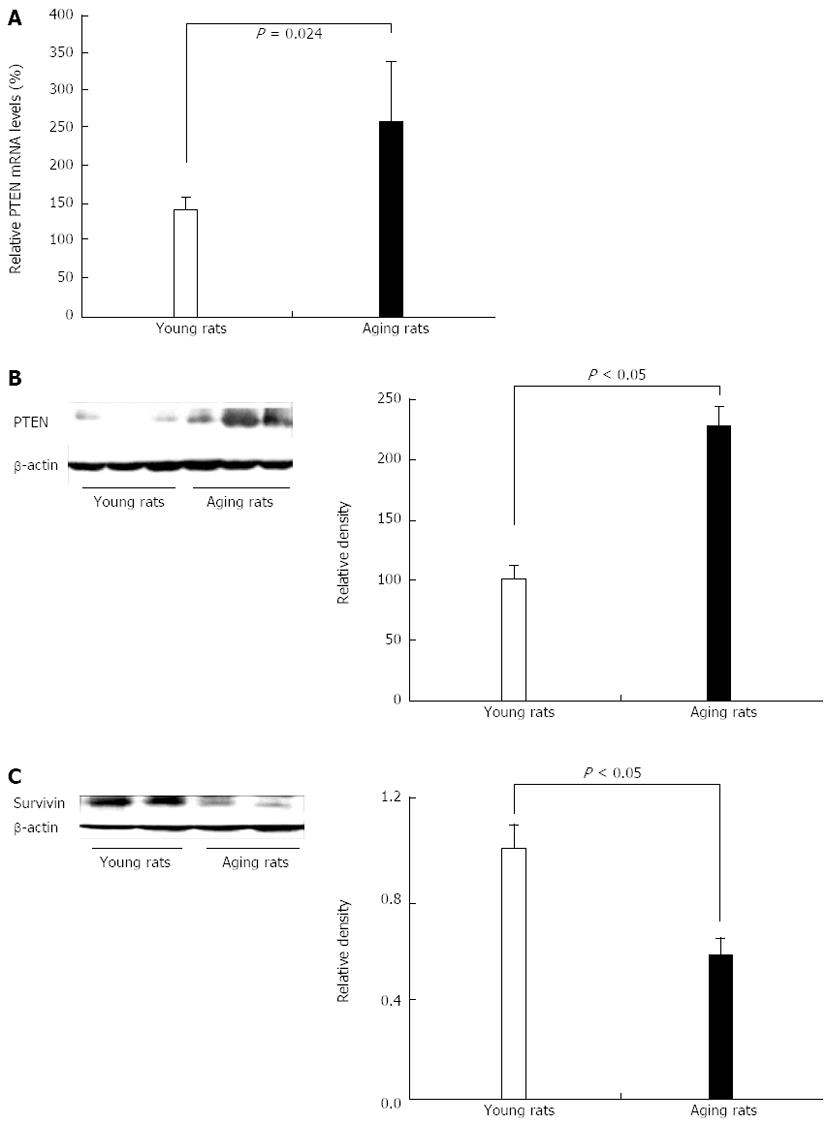
Figure 8 Increased expression of dual phosphatase PTEN and reduced expression of survivin in gastric mucosa of aging vs young rats.
A: Real time PCR; B: Representative Western blotting showing a significant increase in phosphatase and tensin homologue deleted on chromosome ten (PTEN) mRNA and protein expression, respectively in gastric mucosa of aging vs young rats; C: Representative Western blotting showing a significant decrease in survivin (anti-apoptosis protein). Reproduced with permission from Tarnawski et al[1].
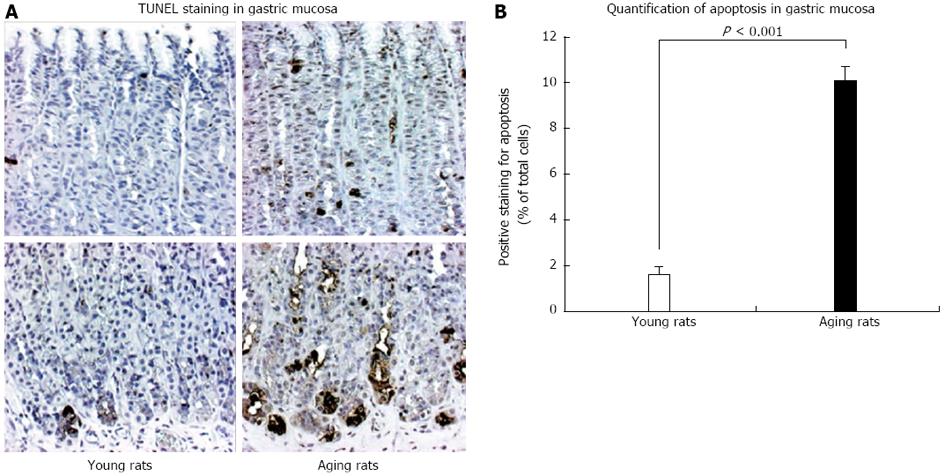
Figure 9 TUNEL staining for apoptosis in gastric mucosa of young and aging rats.
A: The photomicrographs of gastric mucosa of young and aging rats at baseline (magnification x 100). In situ cell death (apoptosis) detection by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was used to visualize apoptotic-positive cells (brown staining); B: Quantification of the number of positively labeled cells demonstrated that gastric mucosa of aging rats exhibits a significantly increased number of apoptotic cells vs mucosa of young rats. The increased apoptosis prominently involved epithelial cells at the basal mucosa explaining atrophy of the basal gastric glands shown in Figure 4. Reproduced with permission from Tarnawski et al[1].
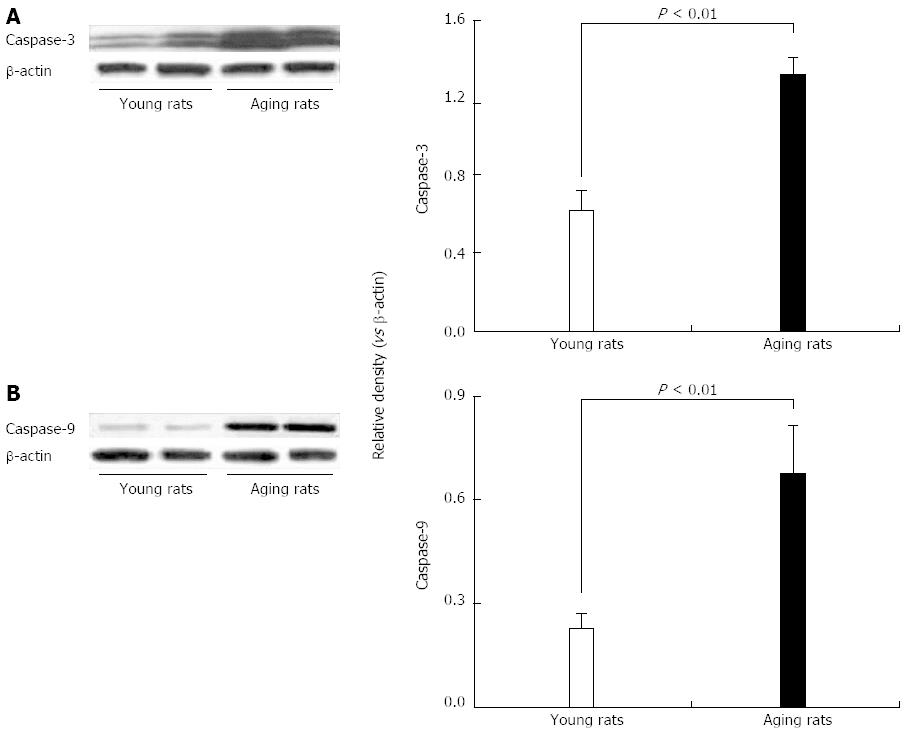
Figure 10 Expression of cleaved caspase-3 and caspase-9 protein levels by Western blotting in gastric mucosa of young and aging rats.
In gastric mucosa of aging rats there is a significant increase in apoptotic cis-inducing (A) cleaved caspase-3 and (B) caspase-9 compared to gastric mucosa of young rats. Reproduced with permission from Tarnawski et al[1].
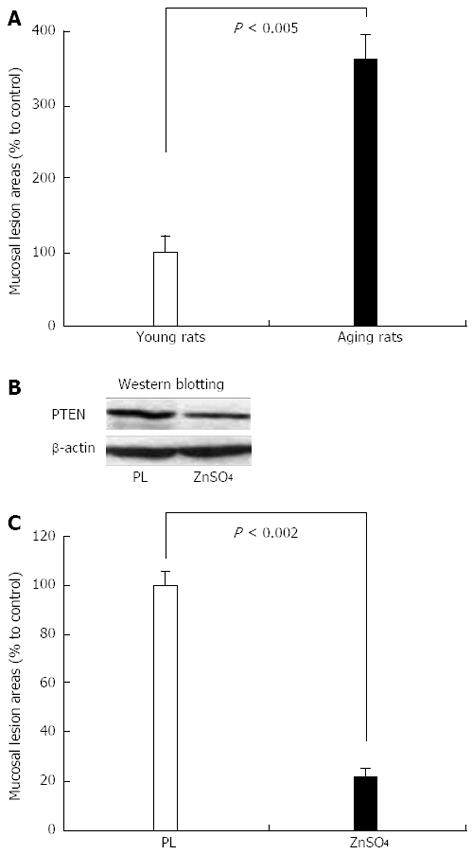
Figure 11 Extent of ethanol-induced gastric mucosal injury in young and aging rats.
A: Three hours after intragastric administration of 8 mL/kg of 50% ethanol, gastric mucosal injury is significantly increased in aging rats vs young rats; B: Intragastric administration of ZnSO4 for 4 h (2 mL 0.5% solution) downregulates phosphatase and tensin homologue deleted on chromosome ten (PTEN) protein expression in gastric mucosa of aging rats vs placebo control (PL); C: Intragastric administration of ZnSO4 to aging rats for 4 h completely reverses the increased susceptibility of gastric mucosa to ethanol-induced injury indicating a causal relationship between PTEN and mucosal injury. Reproduced with permission from Tarnawski et al[1].
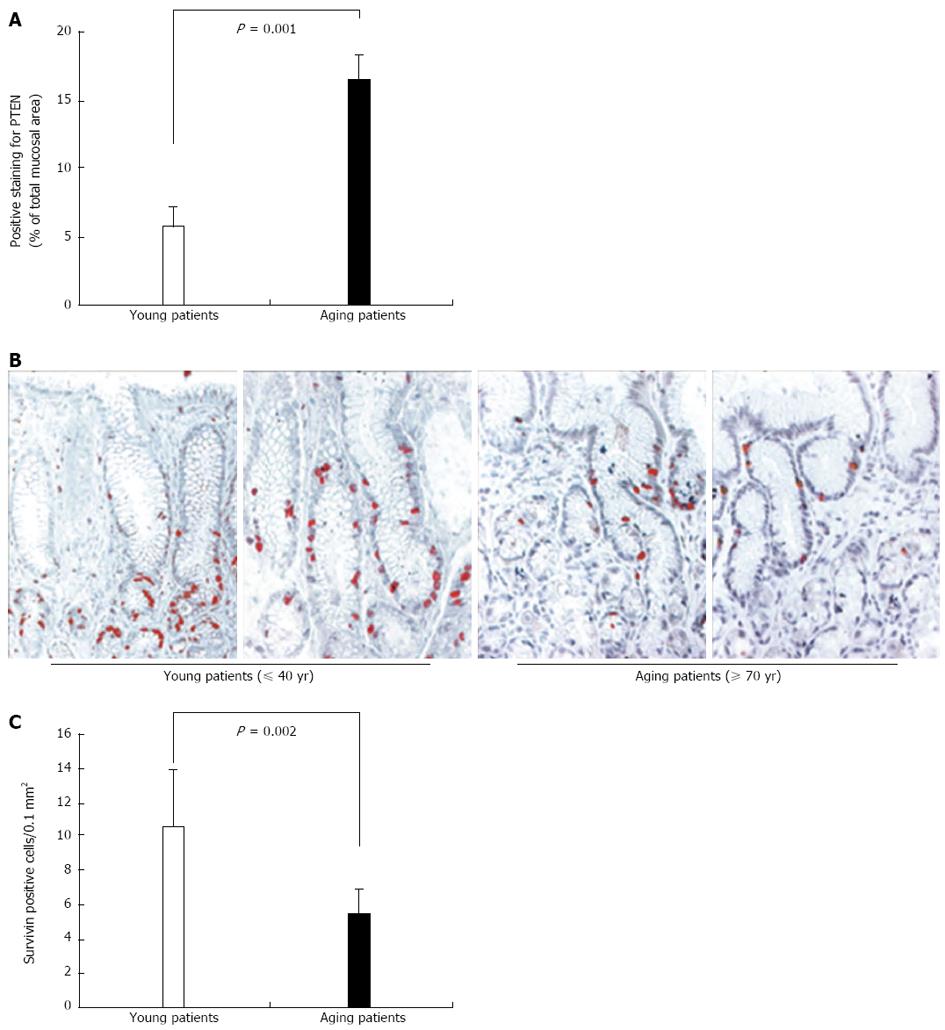
Figure 12 Human relevance: expression of phosphatase and tensin homologue deleted on chromosome ten and survivin in gastric mucosa of young and aging individuals.
A: Quantification of phosphatase and tensin homologue deleted on chromosome ten (PTEN)-positive cells in gastric mucosal sections demonstrated a significantly increased number and significantly increased mucosal area of positively stained cells, mainly epithelial cells in aging (≥ 70 years of age) vs young patients. The threshold of positive staining was set at level 75 on scale 0-255. Reproduced with permission from Tarnawski et al[1]; B: Photomicrographs of representative sections of human gastric mucosa of young and aging patients immunostained for survivin[1]. In gastric mucosa of young patients (40 years of age and younger), survivin expression is strong (brown-red staining) in the nuclei of the progenitor cells; C: Quantification of the number of positively stained cells for survivin demonstrated significantly reduced survivin expression in the gastric mucosa of aging patients (70 years of age or older) vs young patients as reflected by significantly fewer positively stained cells. Reproduced with permission from Tarnawski et al[1].
- Citation: Tarnawski AS, Ahluwalia A, Jones MK. Increased susceptibility of aging gastric mucosa to injury: The mechanisms and clinical implications. World J Gastroenterol 2014; 20(16): 4467-4482
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4467.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4467