©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Nov 28, 2013; 19(44): 7852-7866
Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.7852
Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.7852
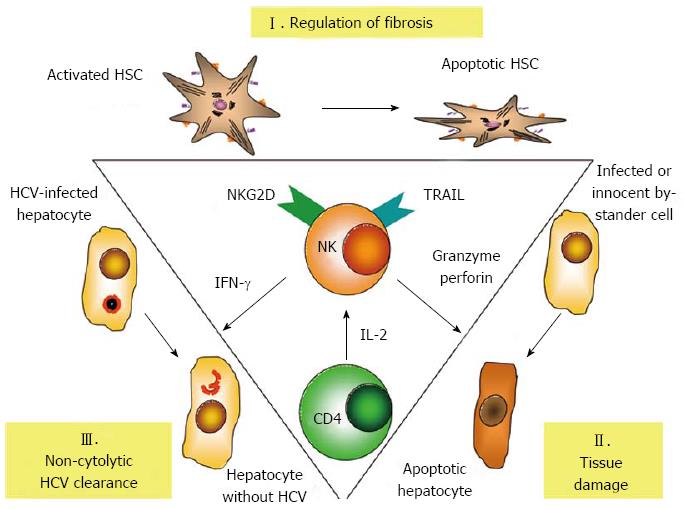
Figure 1 Central role of natural killer cells in the pathogenesis of hepatitis C.
Natural killer (NK) cells regulate fibrosis by killing of activated hepatic stellate cells (HSC), which trigger NK cell activation via natural killer cell receptor with extracellular C-type lectin domains (NKG2D) signalling. The release of granzyme/perforin and cytotoxic cytokines, such as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induce tissue damage. Interferon-γ (IFN-γ) released from NK cells can clear hepatitis C virus (HCV) infection from infected hepatocytes without cytolysis. On the other hand NK cell activity is critically dependent on sufficient supply with interleukin 2 (IL-2) from CD4+ T cells.
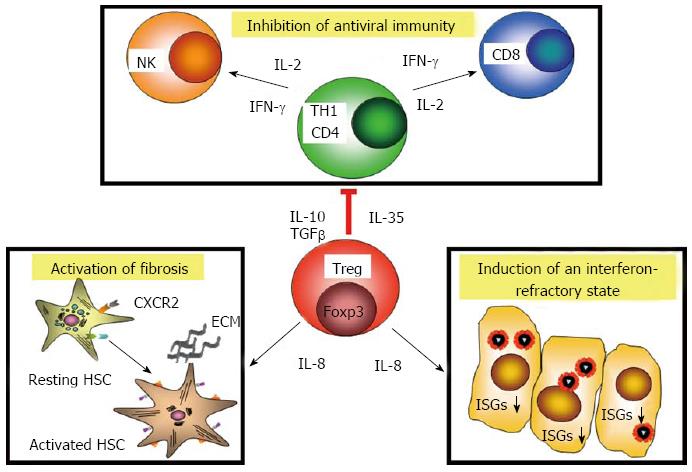
Figure 2 Multiple activities of hepatitis C virus-specific regulatory T cells in chronic hepatitis C.
Regulatory T cell (Tregs) inhibit antiviral immunity via release of immunosuppressive factors, such as interleukin 10 (IL-10), transforming growth factor beta (TGF-β) and interleukin 35 (IL-35). Tregs in hepatitis C are also differentiated towards interleukin 8 (IL-8) production. Release of IL-8 binds to its receptors, such as CXCR2 on hepatic stellate cells (HSC), which become activated and produce extracellular matrix (ECM) components. IL-8 down-regulates interferon-stimulated genes in infected cells and induces an interferon (IFN)-refractory state, which also counter-acts antiviral immunity. ISGs: Interferon-stimulated genes.
- Citation: Spengler U, Nischalke HD, Nattermann J, Strassburg CP. Between Scylla and Charybdis: The role of the human immune system in the pathogenesis of hepatitis C. World J Gastroenterol 2013; 19(44): 7852-7866
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/7852.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.7852