Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 14, 2013; 19(10): 1551-1562
Published online Mar 14, 2013. doi: 10.3748/wjg.v19.i10.1551
Published online Mar 14, 2013. doi: 10.3748/wjg.v19.i10.1551
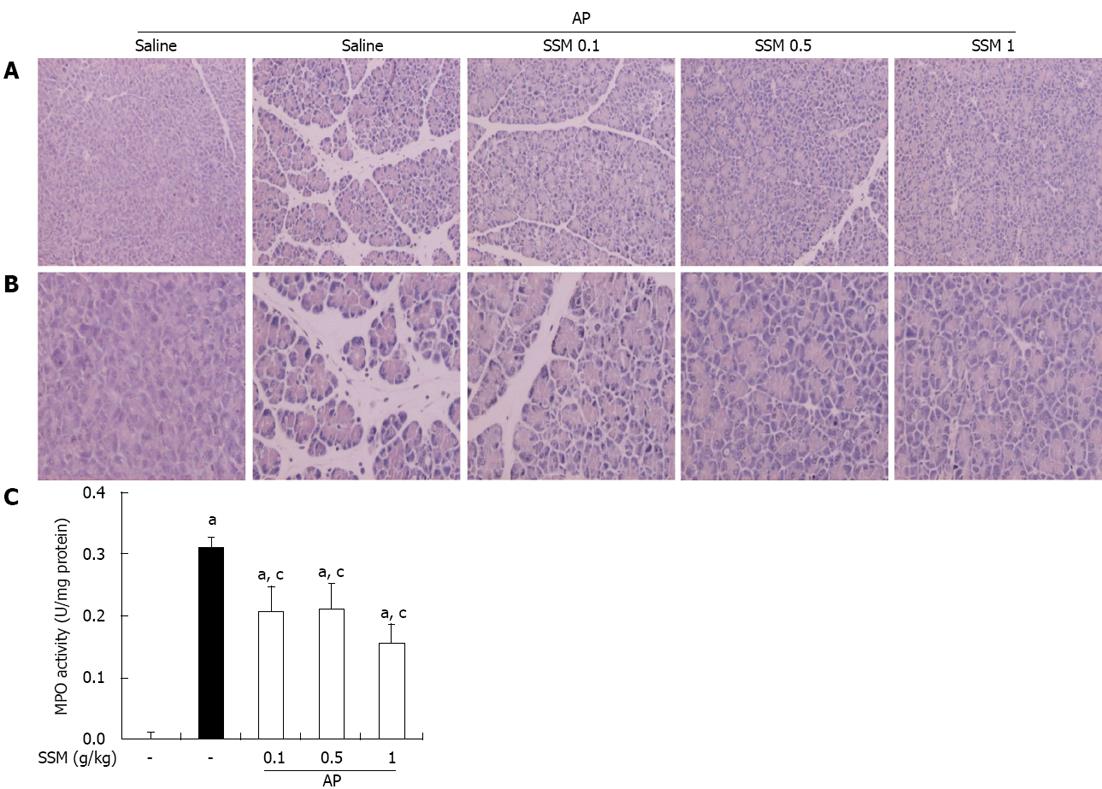
Figure 1 Effects of Scolopendra subspinipes mutilans on inflammation in the pancreas following pancreatitis.
A, B: 200 × (A) and 400 × (B) magnification of representative hematoxylin and eosin stained pancreatic sections of control mice and mice pretreated with Scolopendra subspinipes mutilans (SSM) (0.1, 0.5 or 1 g/kg) 1 h before the cerulein (50 μg/kg)-mediated induction of acute pancreatitis (AP); C: Myeloperoxidase (MPO) activity was measured in the pancreas 6 h after completion of the cerulein injections. Data are expressed as U/mg protein. Data are represented as mean ± SE (n = 6 in each group). The results were similar in 3 further experiments. aP < 0.05 vs control group, cP < 0.05 vs cerulein treatment alone.
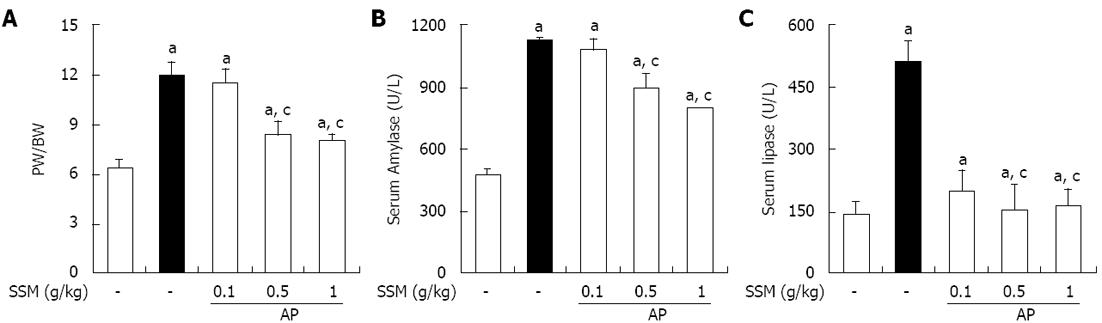
Figure 2 Effects of Scolopendra subspinipes mutilans pretreatment on the pancreatic weight/body weight ratio and the production of digestive enzymes such as serum amylase and serum lipase during cerulein-induced acute pancreatitis.
Mice pretreated with Scolopendra subspinipes mutilans (SSM) (0.1, 0.5 or 1 g/kg) were challenged with intraperitoneal injections of cerulein (50 μg/kg). Mice were sacrificed 6 h after the last cerulein injection. Serum and pancreas were harvested and the pancreatic weight (PW)/body weight (BW) (A) and levels of digestive enzymes such as amylase (B) and lipase (C) were measured as indicated in the experimental protocol. Data are represented as mean ± SE (n = 6 in each group). The results were similar in 3 further experiments. aP < 0.05 vs control group, cP < 0.05 vs cerulein treatment alone. AP: Acute pancreatitis.
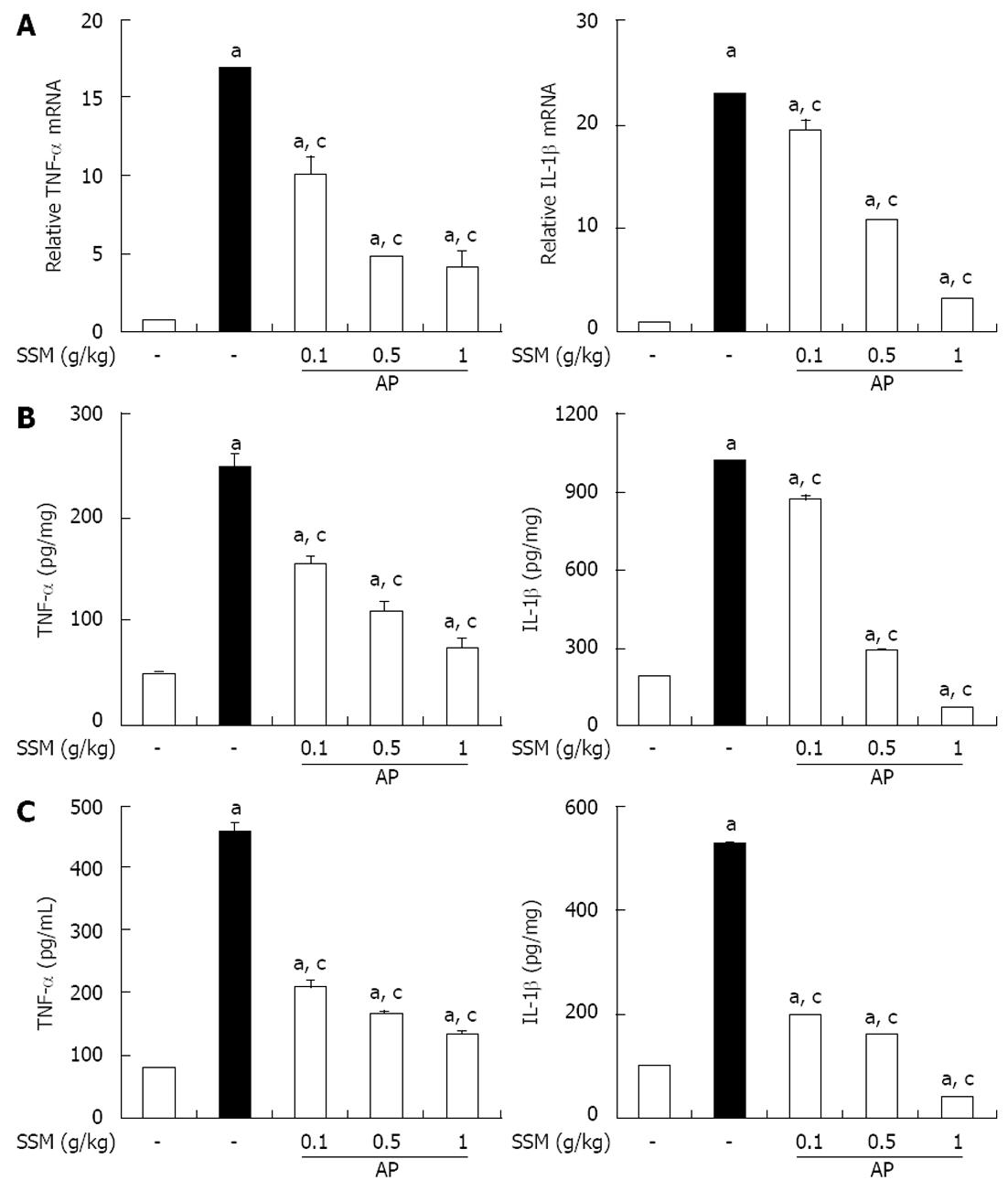
Figure 3 Effect of Scolopendra subspinipes mutilans on tumor necrosis factor-α and interleukins-1β during cerulein-induced acute pancreatitis.
Mice pretreated with Scolopendra subspinipes mutilans (SSM) (0.1, 0.5, or 1 g/kg) were challenged with intraperitoneal injections of cerulein at a supramaximal dose (50 μg/kg). Mice were sacrificed 6 h after the last cerulein injection. A-C: Levels of pancreatic tumor necrosis factor (TNF)-α and interleukin (IL)-1β mRNA were quantified by real-time reverse transcriptase-polymerase chain reaction (A) and the corresponding protein levels were measured in the pancreatic tissue (B) and serum by enzyme-linked immunosorbent assay (C). Data are represented as mean ± SE (n = 6 in each group). The results were similar in 3 further experiments. aP < 0.05 vs control group, cP < 0.05 vs cerulein treatment alone. AP: Acute pancreatitis.
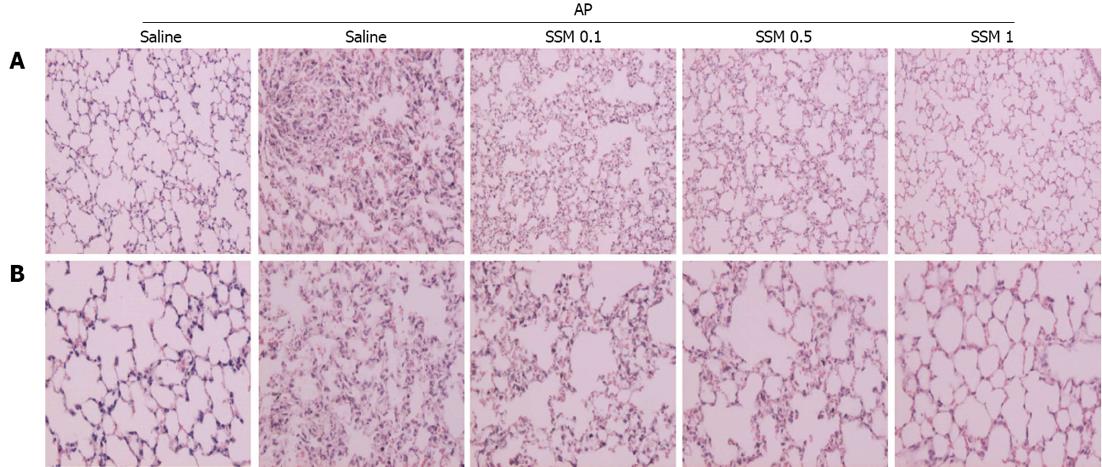
Figure 4 Effects of Scolopendra subspinipes mutilans on acute pancreatitis-associated lung injury.
A, B: 200 × (A) and 400 × (B) magnification of representative hematoxylin and eosin-stained lung sections of control mice and mice pretreated with Scolopendra subspinipes mutilans (0.1, 0.5, or 1 g/kg) 1 h before the cerulein (50 μg/kg)-mediated induction of acute pancreatitis. Data are represented as mean ± SE (n = 6 in each group). The results were similar in 3 additional experiments.
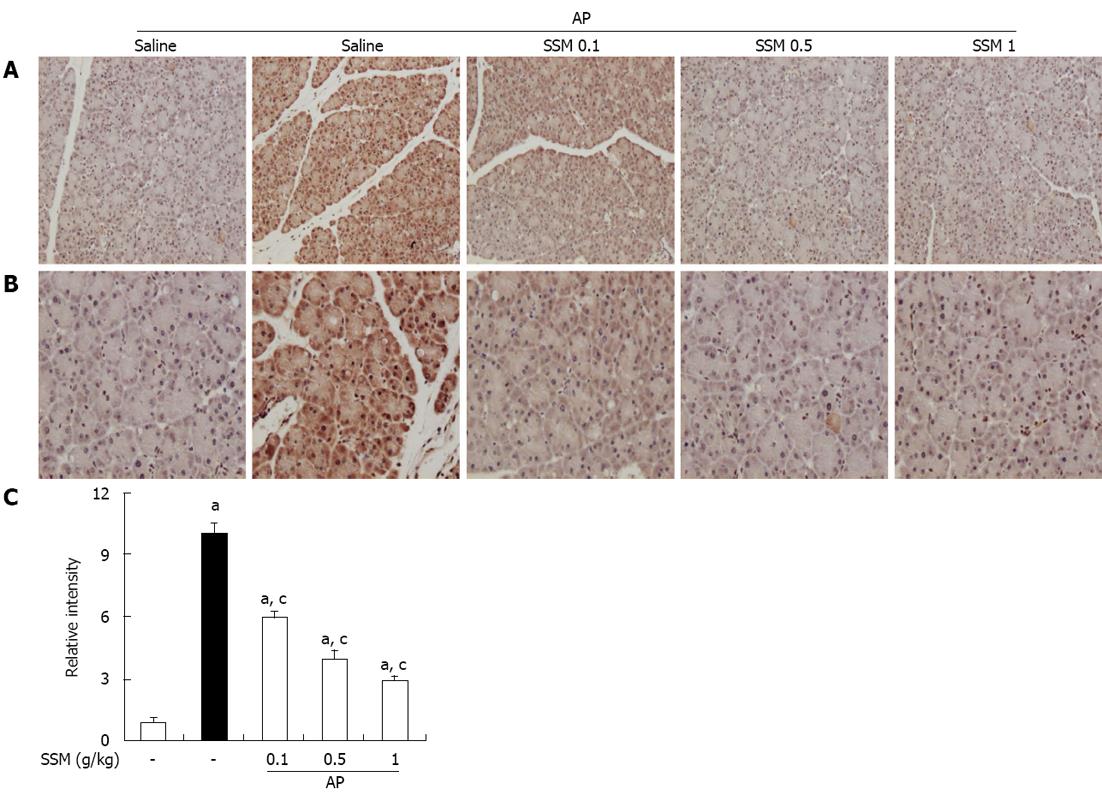
Figure 5 Effects of Scolopendra subspinipes mutilans on pancreatic high-mobility group box protein-1 expression in cerulein-induced acute pancreatitis.
A, B: 200 × (A) and 400 × (B) magnification of representative immunohistochemical data, detecting high-mobility group box protein-1 (HMGB-1) expression in pancreatic tissue sections of control mice and mice pretreated with Scolopendra subspinipes mutilans (SSM) (0.1, 0.5, or 1 g/kg) 1 h before the cerulein (50 μg/kg)-mediated induction of acute pancreatitis (AP). Mice were sacrificed 6 h after the last cerulein injection; C: Relative intensity of HMGB-1 staining was scored as described in Materials and Methods. Data are represented as mean ± SE (n = 6 in each group). The results were similar in 3 further experiments. aP < 0.05 vs control group, cP < 0.05 vs cerulein treatment alone.
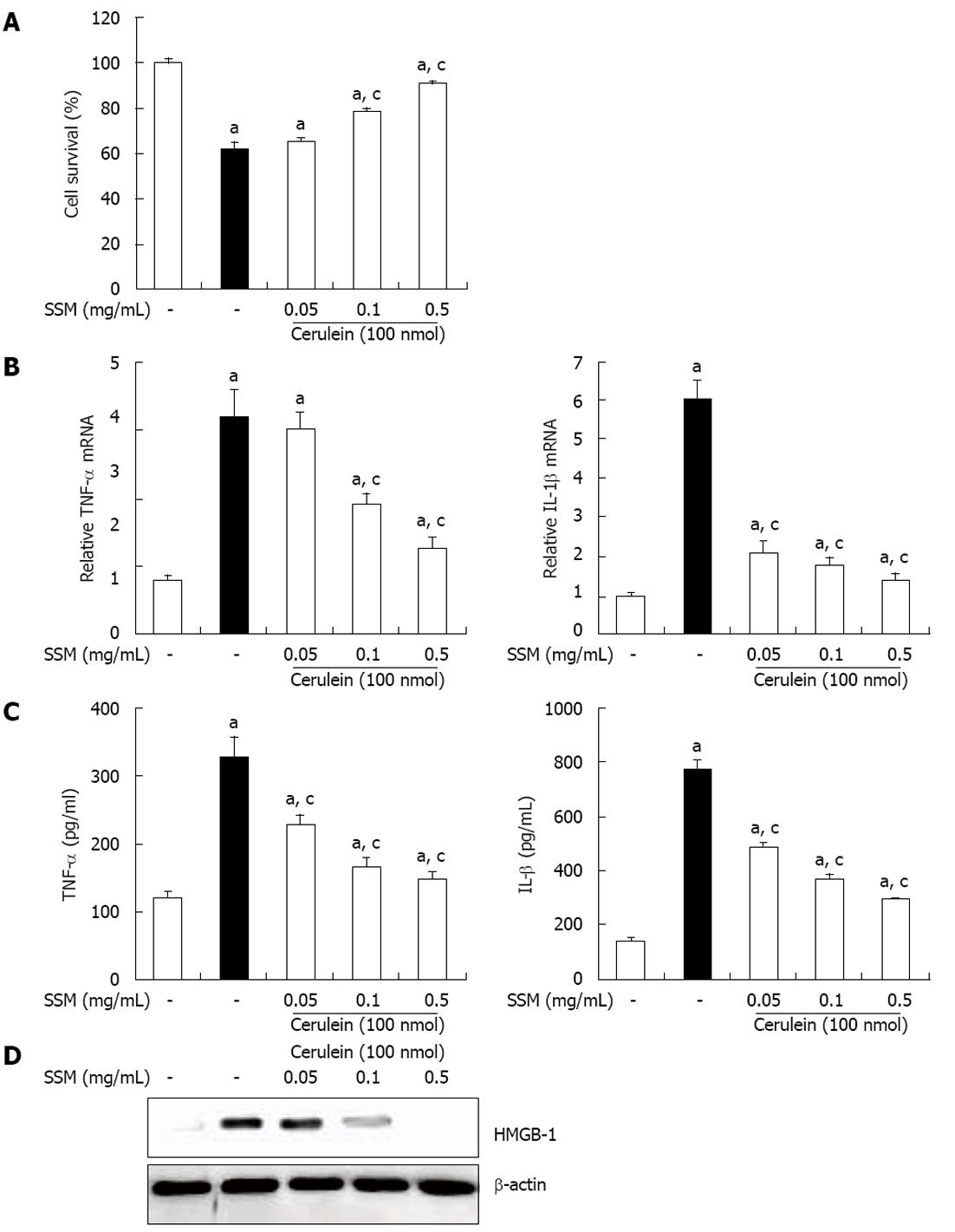
Figure 6 Effect of Scolopendra subspinipes mutilans on cerulein-induced acinar cell death and the production of inflammatory mediators.
The acinar cells were pretreated with Scolopendra subspinipes mutilans (SSM) for 1 h at indicated doses. A: 6 h after cerulein stimulation, the cell viability was measured; B, C: After 24 h of cerulein stimulation, cytokine levels in isolated pancreatic acinar cells were examined using enzyme-linked immunosorbent assay (B) and real-time reverse transcription polymerase chain reaction (C); D: And also the high-mobility group box protein-1 (HMGB-1) levels were measured by western blot. Data are represented as mean ± SE (n = 6 in each group). The results were similar in 3 further experiments. aP < 0.05 vs control group, cP < 0.05 vs cerulein treatment alone. TNF: Tumor necrosis factor; IL: Interleukins.
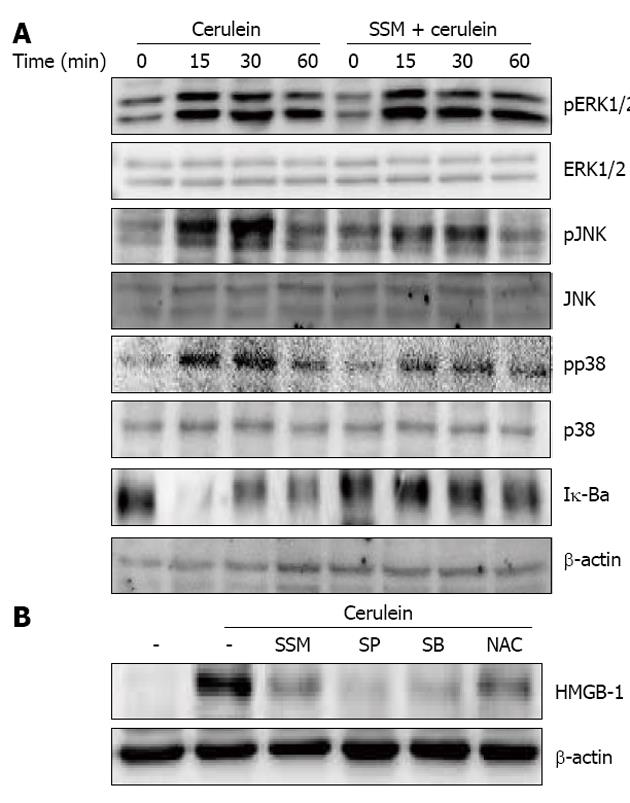
Figure 7 Effect of Scolopendra subspinipes mutilans on activation of mitogen activated protein kinases and nuclear factor-κB.
A: Isolated pancreatic acinar cells were pretreated with Scolopendra subspinipes mutilans (SSM) for 1 h and then stimulated with cerulein for indicated times. The expression of phosphospecific mitogen activated protein kinases and degradation of Iκ-Bα in pancreatic acini was examined via western blot; B: The acinar cells were pretreated with c-Jun NH2-terminal kinase (JNK) inhibitor (SP600125 50 μmol), p38 inhibitor (SB239063 20 μmol), nuclear factor-κB inhibitor (N-acetyl cystein, NAC, 10 mmol) or SSM (0.5 mg/mL) for 1 h. Then cerulein was added into isolated pancreatic acinar cells and incubated for 24 h. High-mobility group box protein-1 (HMGB-1) expression was measured via western blot. The results were similar in 3 further experiments. ERK: Extracellular signal-related kinase.
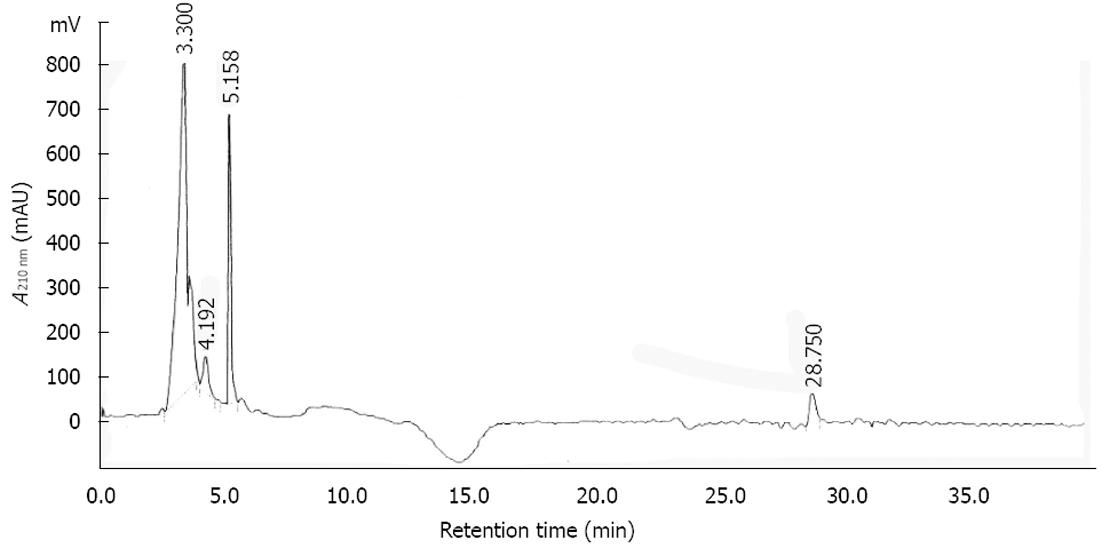
Figure 8 High-performance liquid chromatography chromatogram of the Scolopendra subspinipes mutilans at the length of 210 nm.
-
Citation: Jo IJ, Bae GS, Park KC, Choi SB, Jung WS, Jung SY, Cho JH, Choi MO, Song HJ, Park SJ.
Scolopendra subspinipes mutilans protected the cerulein-induced acute pancreatitis by inhibiting high-mobility group box protein-1. World J Gastroenterol 2013; 19(10): 1551-1562 - URL: https://www.wjgnet.com/1007-9327/full/v19/i10/1551.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i10.1551