©2010 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 14, 2010; 16(46): 5889-5894
Published online Dec 14, 2010. doi: 10.3748/wjg.v16.i46.5889
Published online Dec 14, 2010. doi: 10.3748/wjg.v16.i46.5889
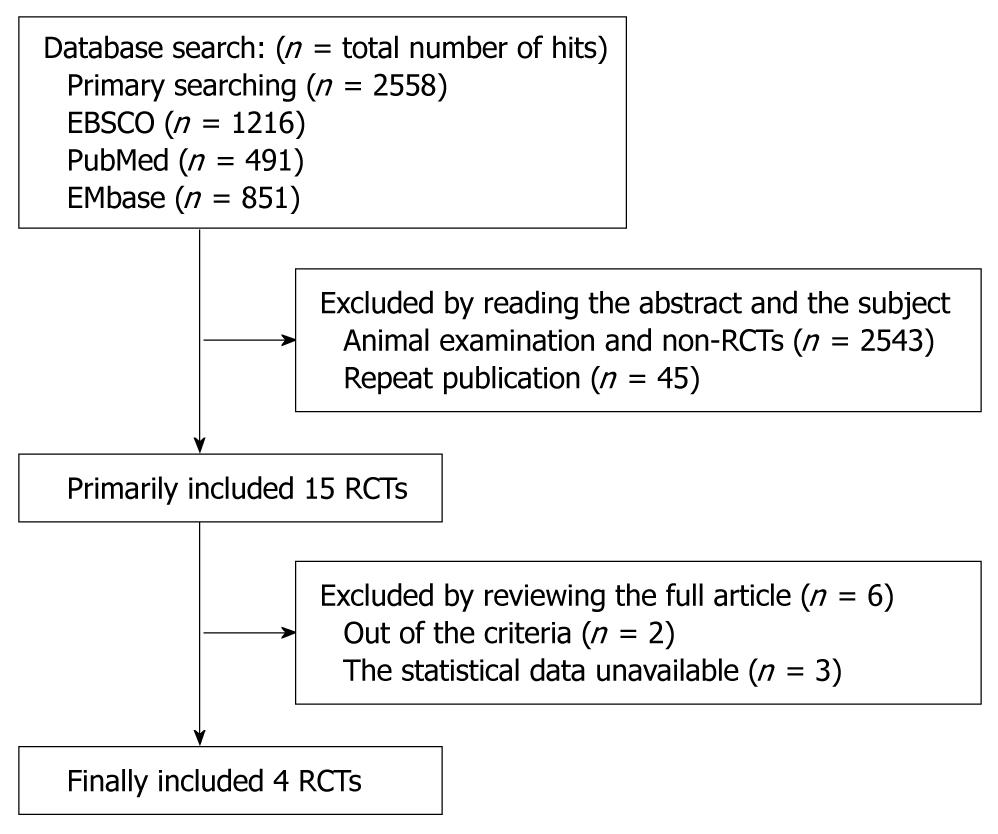
Figure 1 Article search and riddling progression.
RCTs: Randomized controlled trials.
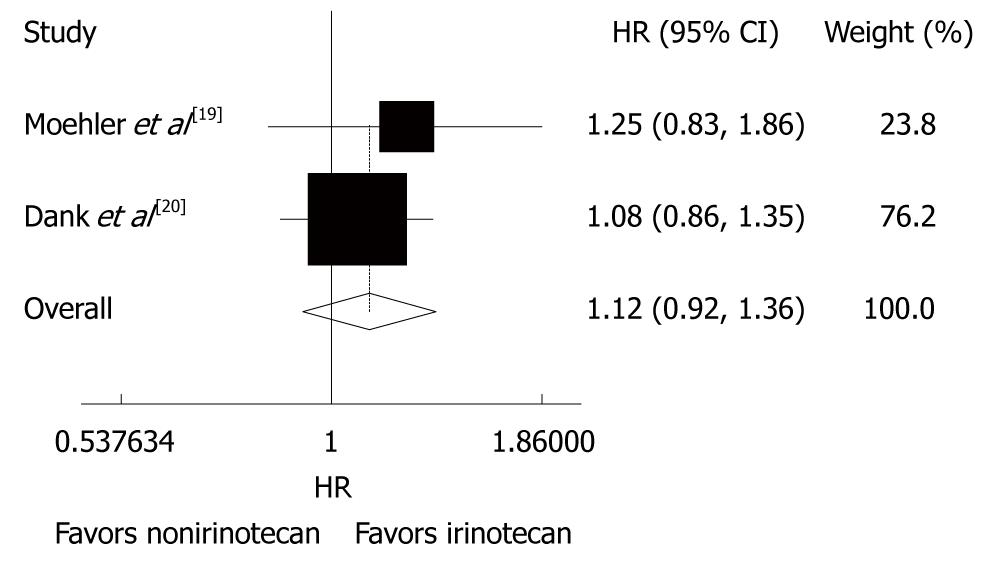
Figure 2 Overall survival rate of patients with advanced gastric cancer after irinotecan-containing and nonirinotecan-containing combination chemotherapies.
HR: Hazard ratio.
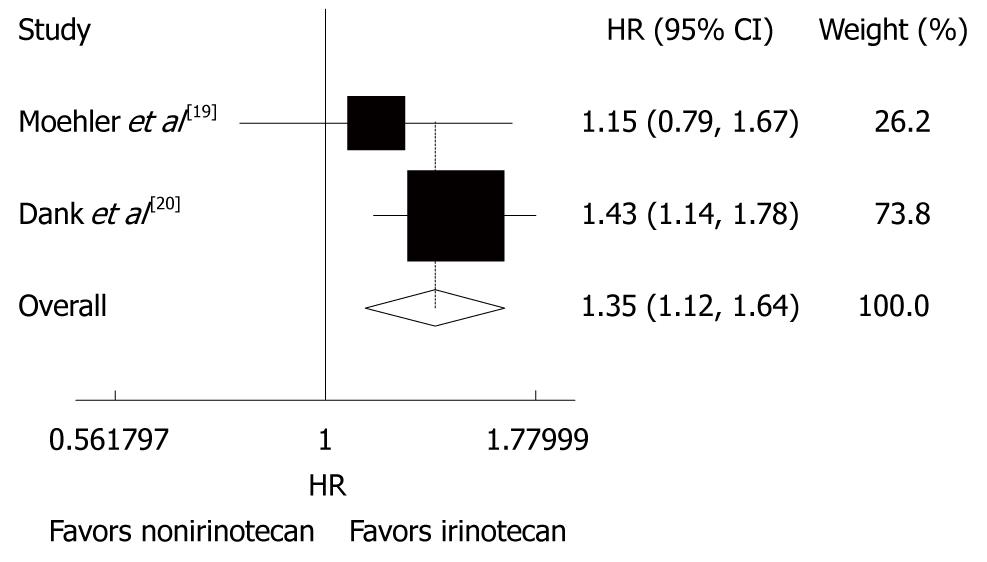
Figure 3 Time to treatment failure of patients with advanced gastric cancer irinotecan-containing and nonirinotecan-containing combination chemotherapies.
HR: Hazard ratio.
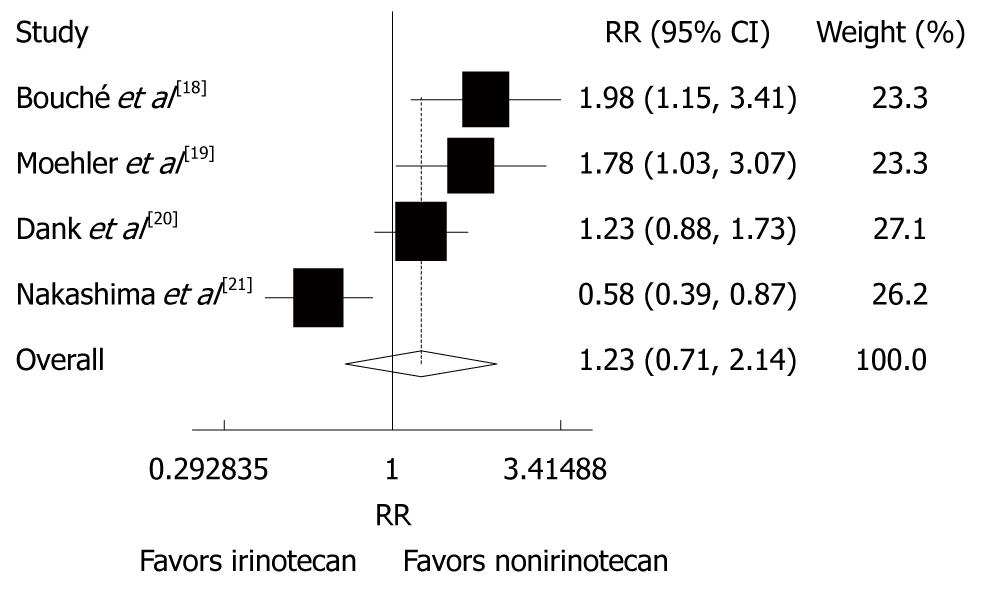
Figure 4 Overall response rate of patients with advanced gastric cancer after irinotecan-containing and nonirinotecan-containing combination chemotherapies.
RR: Risk ratio.
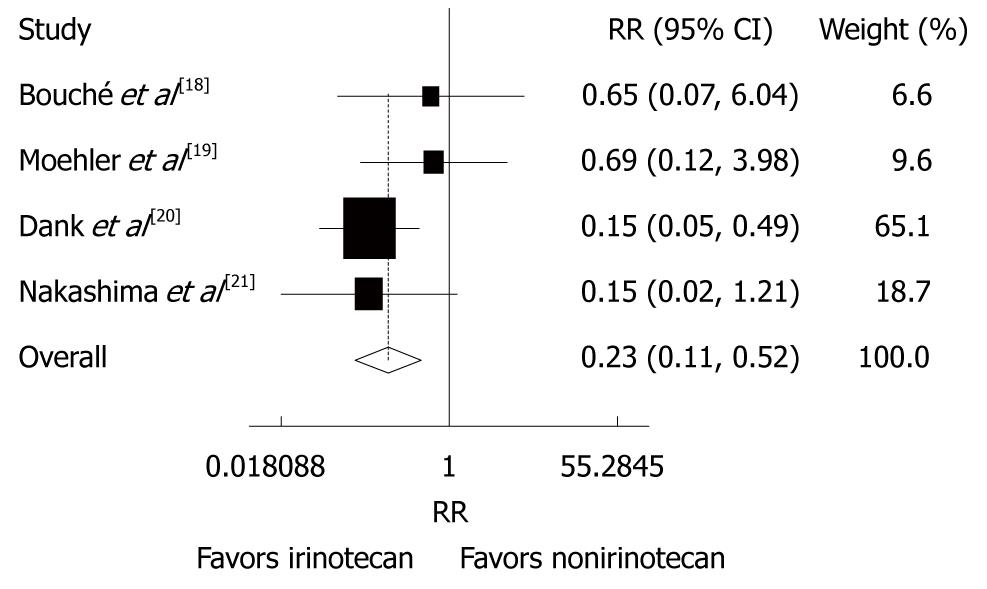
Figure 5 Grade 3/4 haematological toxicities (thrombocytopenia) in patients with advanced gastric cancer after irinotecan-containing and nonirinotecan-containing combination chemotherapies.
RR: Risk ratio.
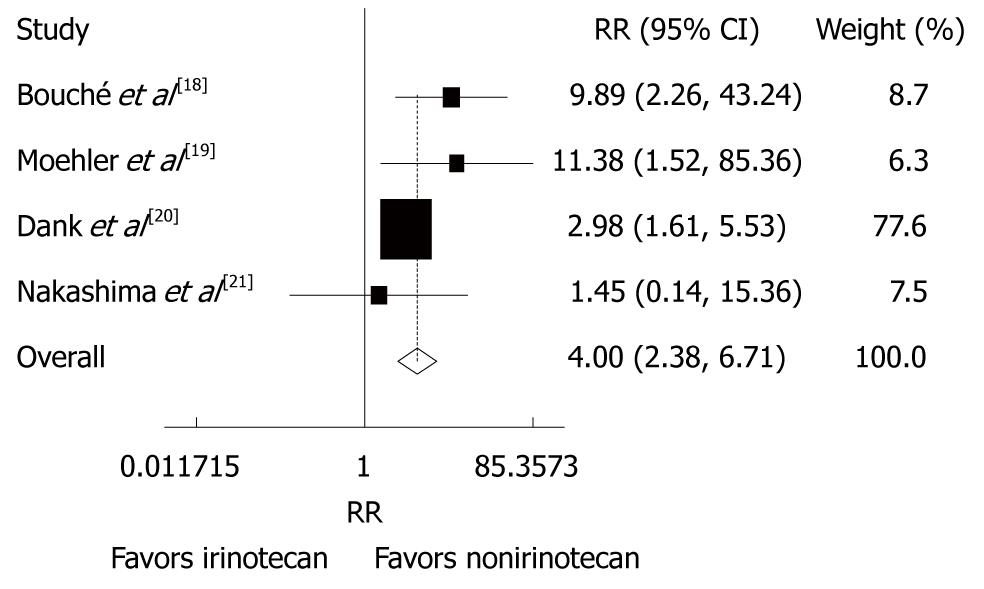
Figure 6 Grade 3/4 gastrointestinal toxicities (diarrhea) in patients with advanced gastric cancer after irinotecan-containing and nonirinotecan-containing combination chemotherapies.
RR: Risk ratio.
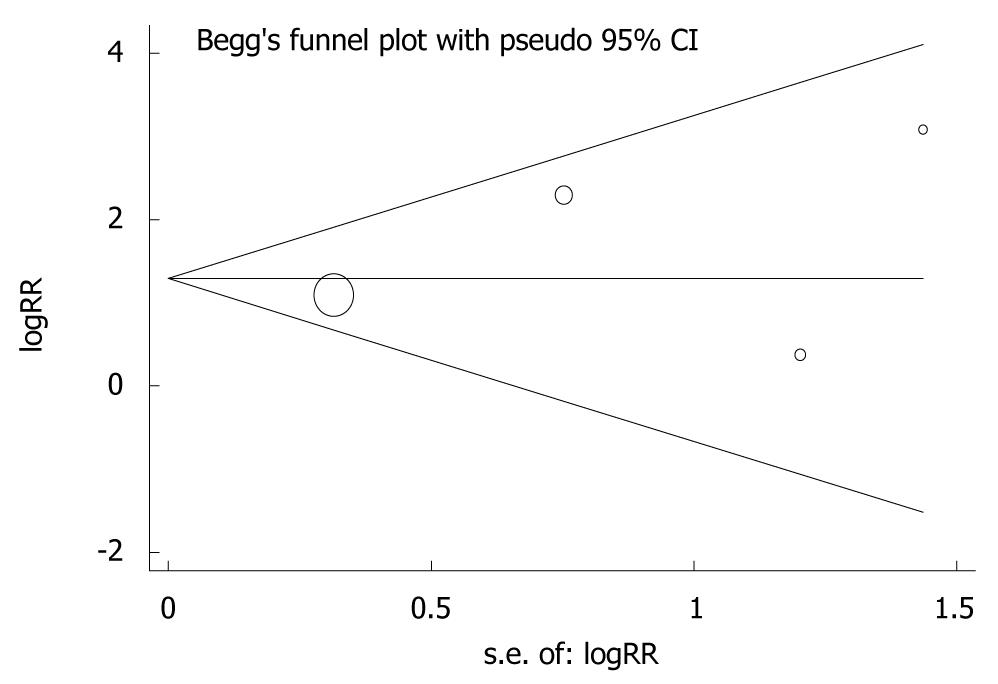
Figure 7 Funnel plots and Egger’s linear regression test showing the potential publication bias in diagnosis of advanced gastric cancer[22].
RR: Risk ratio.
- Citation: Wang DL, Gu DY, Huang HY, Xu Z, Chen JF. Irinotecan-involved regimens for advanced gastric cancer: A pooled-analysis of clinical trials. World J Gastroenterol 2010; 16(46): 5889-5894
- URL: https://www.wjgnet.com/1007-9327/full/v16/i46/5889.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i46.5889