©2010 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 14, 2010; 16(46): 5779-5789
Published online Dec 14, 2010. doi: 10.3748/wjg.v16.i46.5779
Published online Dec 14, 2010. doi: 10.3748/wjg.v16.i46.5779
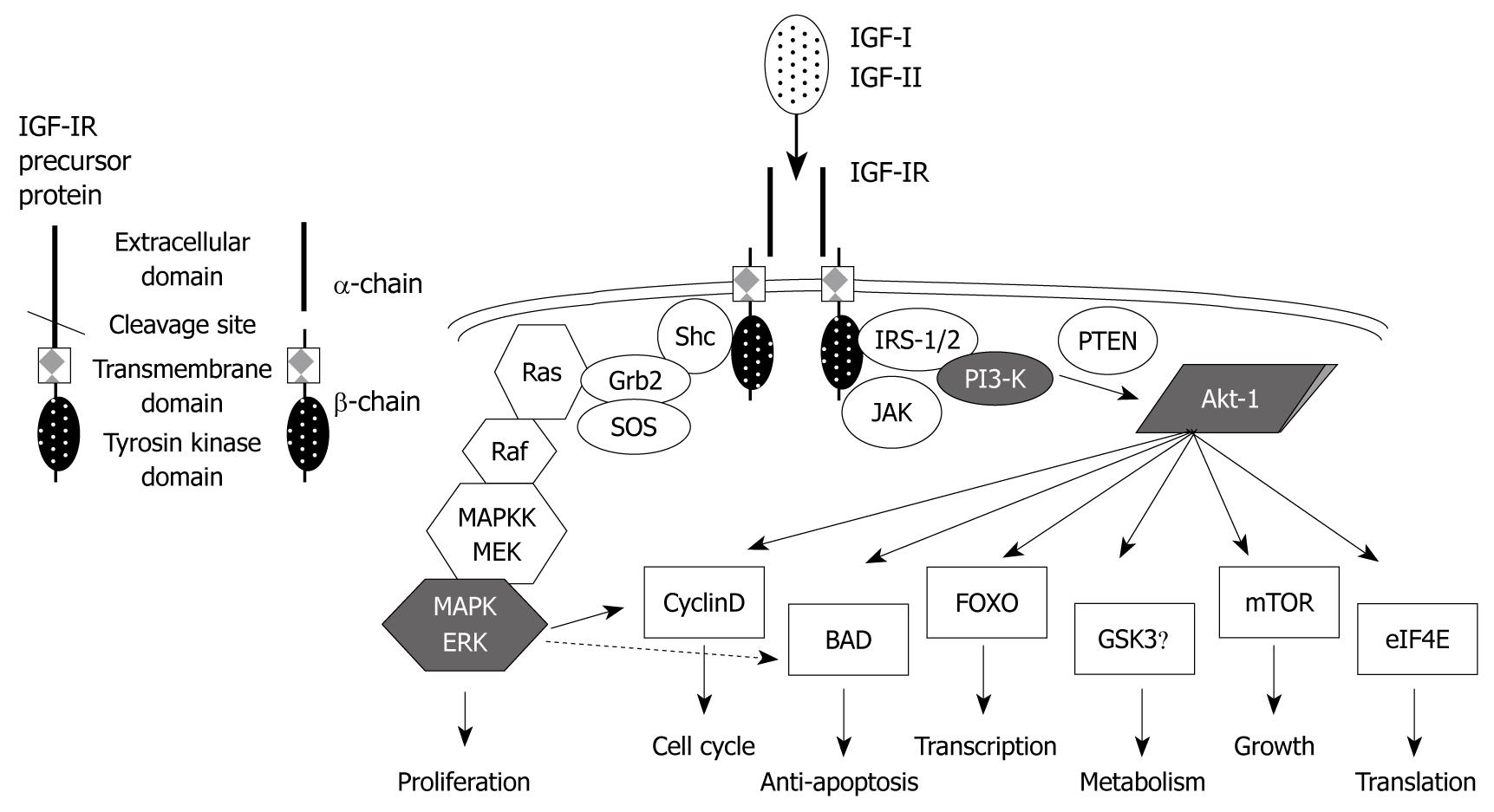
Figure 1 The structure, signal transductions, and effects of the type I insulin-like growth factor receptor system.
Type I insulin-like growth factor receptor (IGF-IR) is synthesized as a single precursor peptide and then is cleaved into the α-subunit (extracellular domain) and the β-subunit (transmembrane and tyrosine kinase domains). After binding to the ligands (IGF-I and IGF-II), IGF-IR, which is constructed with two α- and two β-chains, turns on its signal transductions via two major pathways, such as mitogen-activated protein kinase (MAPK) and phosphatidylinositide 3-kinase (PI3-K)/Akt, results in survival and mitogenesis. IRS: Insulin receptor substrate; Shc: Src homology and collagen-containing protein; Grb2: Growth factor receptor-bound protein 2; PTEN: Phosphatase and tensin homolog; JAK: Janus kinase; MAPKK: MAPK kinase; MEK: MAPK/ERK kinase; ERK: Extracellular signal-regulated kinase; BAD: Bcl-2-associated death promoter; FOXO: Forkhead box O; GSK3β: Glycogen synthase kinase 3 beta; eIF4E: Eukaryotic translation initiation factor 4E.
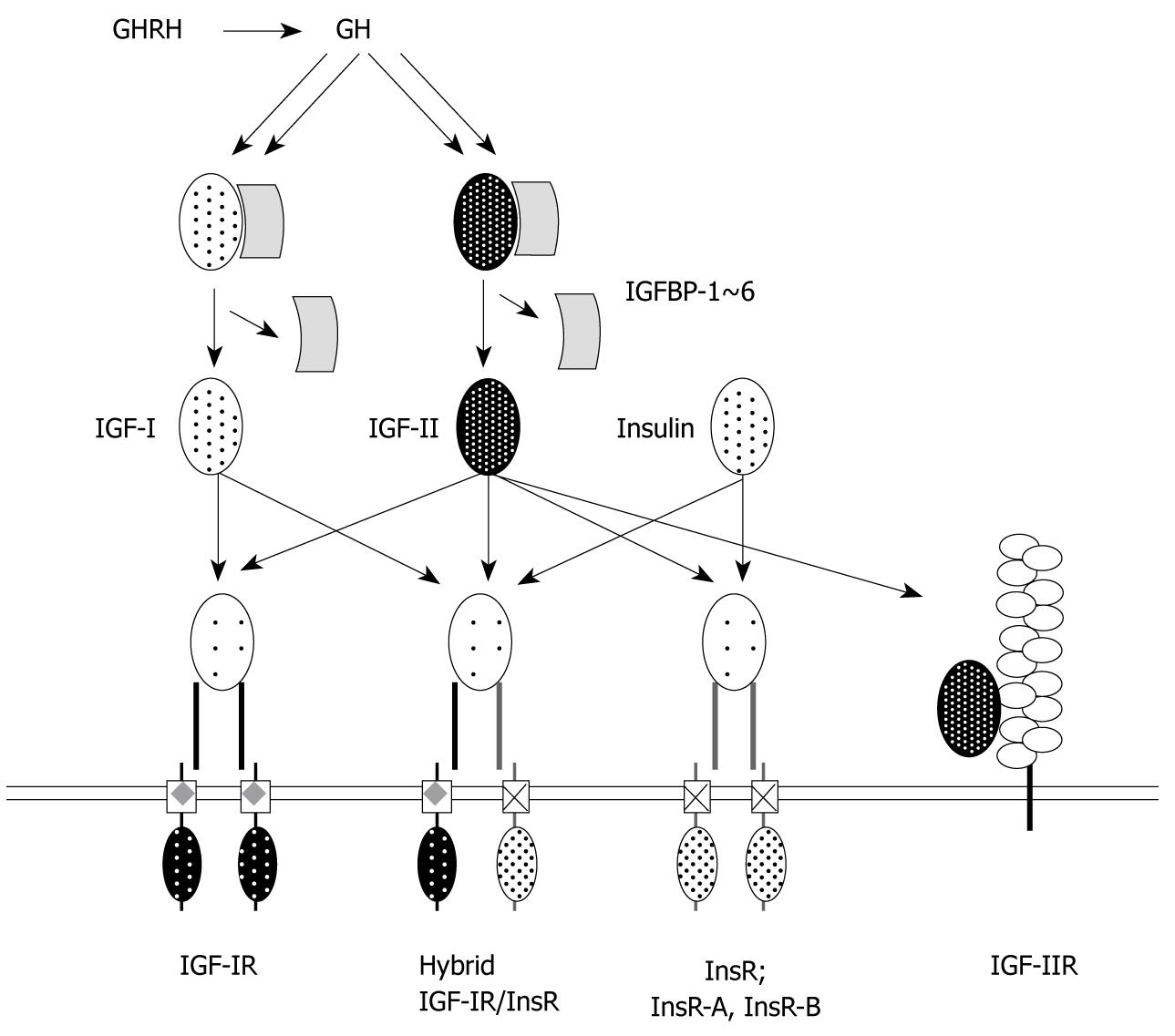
Figure 2 Insulin-like growth factor/type I insulin-like growth factor receptor and insulin/insulin receptor systems.
Growth hormone-releasing hormone (GHRH) can stimulate secretion of growth hormone (GH), which upregulates insulin-like growth factors (IGFs) expression. IGF-I and IGF-II, which have about 40% sequence similarity to pro-insulin, predominantly activate type I IGF receptor (IGF-IR), which is a similar structure to insulin receptor (InsR) (59% sequence similarity). IGF-II is able to bind IR and both IGFs can bind hybrid IGF-IR/IR receptors. Ligand supply of both IGFs is regulated by two components. One is IGF binding proteins, which comprise at least six proteins [IGF binding protein (IGFBP)-1~6]. Another is IGF-IIR (lacks tyrosine kinase activity), which internalizes IGF-II for degradation in the pre-lysosomal compartment. Insulin can activate both IR and hybrid IGF-IR/InsR. Two isoforms of InsR exist, the A-isoform (InsR-A) and the B-isoform (InsR-B).
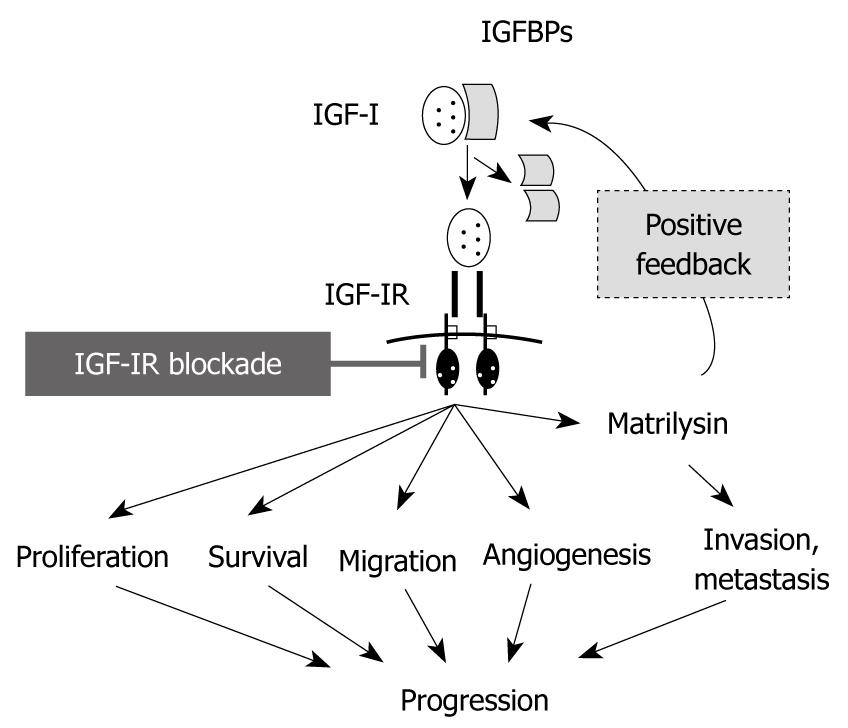
Figure 3 Blockades of type I insulin-like growth factor receptor reduce tumor progression through several interruptions of type I insulin-like growth factor receptor-mediated functions, including type I insulin-like growth factor receptor/matrilysin positive feedback system.
IGF-IR: Type I insulin-like growth factor receptor; IGFBPs: IGF binding proteins.
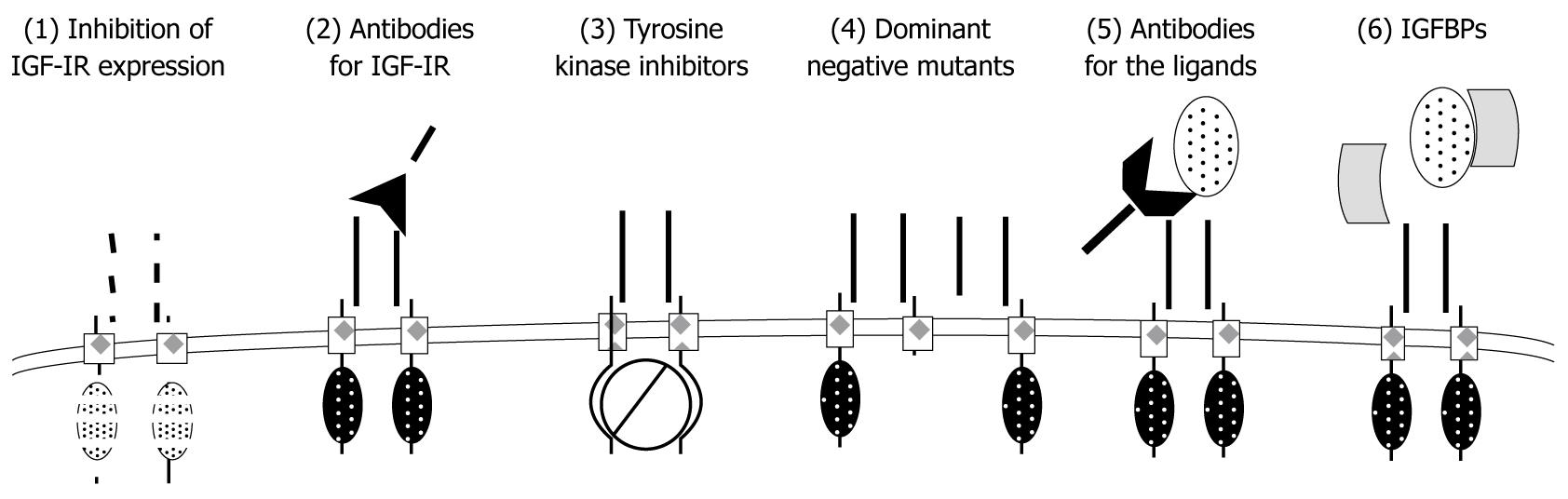
Figure 4 The summary of strategies to inactivate type I insulin-like growth factor receptor system.
(1) Inhibition of type I insulin-like growth factor receptor (IGF-IR) protein expression by blocking its translation [with antisense oligodeoxynucleotides and short interfering RNA (siRNA)]; (2) IGF-IR function can be blocked with inactivating monoclonal antibodies (mAb); (3) The tyrosine kinase activity of IGF-IR can be abolished with small-molecule inhibitors (TKI); (4) IGF-IR mutants lacking β-subunits can act as dominant-negative (dn) receptors; (5) Ligand availability can be reduced by mAb for IGFs; and (6) IGF binding proteins (IGFBPs) can reduce active forms of IGFs.
- Citation: Adachi Y, Yamamoto H, Ohashi H, Endo T, Carbone DP, Imai K, Shinomura Y. A candidate targeting molecule of insulin-like growth factor-I receptor for gastrointestinal cancers. World J Gastroenterol 2010; 16(46): 5779-5789
- URL: https://www.wjgnet.com/1007-9327/full/v16/i46/5779.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i46.5779