©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Feb 21, 2007; 13(7): 1032-1041
Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1032
Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1032
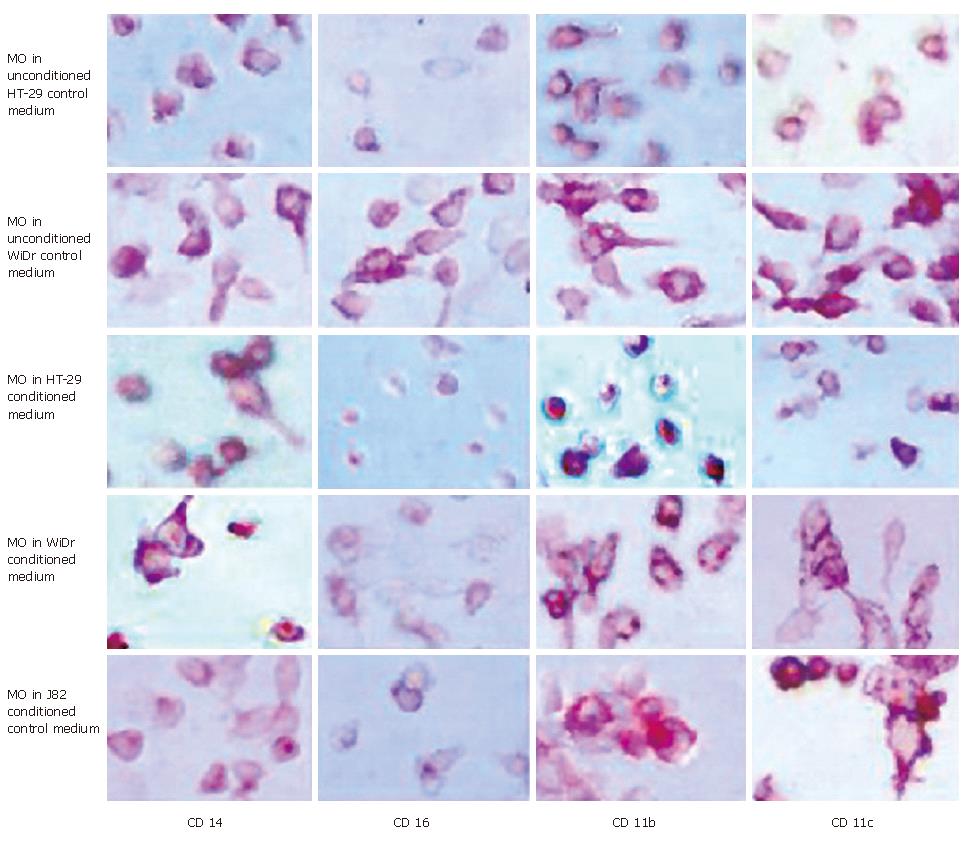
Figure 1 Immunohistochemical detection of MO/MAC antigen expression after 7 d of culture in IEC conditioned medium.
Freshly elutriated MO were incubated in unconditioned control media, conditioned media of the IEC lines HT-29 and WiDr and conditioned medium of the control cell line J82 of non-intestinal origin for seven days. Antigen expression was determined by immunohistochemistry (APAAP-method). Expression of the MO/MAC specific antigens CD14, CD16, CD11b and CD11c was determined. All tested antigens were detectable on the cells after the seven-day culture period. Incubation in IEC-conditioned medium had no influence on antigen expression.
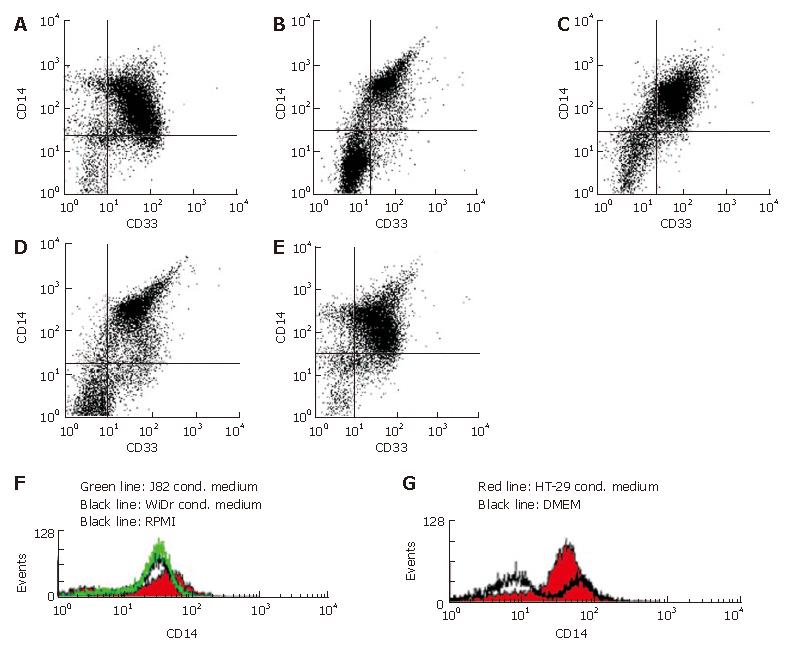
Figure 2 Flow cytometrical quantification of MO/MAC antigen expression after seven days of culture in IEC conditioned medium.
A: Ninety-two percent of CD33+ cells (MO/MAC) showed expression of CD14 in MO cultured in unconditioned control medium (RPMI) without FCS supplemented with 2% human AB serum for seven days; B: Ninety-five percent of CD33+ cells (MO/MAC) were CD14-positiv in MO cultured in WiDr conditioned medium without FCS supplemented with 2% human AB serum for seven days; C: Ninety-two percent of CD33+ cells (MO/MAC) were CD14-positive in MO cultured in J82 conditioned medium (control cell line of non-intestinal origin) without FCS supplemented with 2% human AB serum for seven days; D: Ninety-four percent of CD33+ cells (MO/MAC) showed expression of CD14 in MO cultured in unconditioned control medium (DMEM) without FCS supplemented with 2% human AB serum for seven days; E: Ninety-eight percent of CD33+ cells (MO/MAC) were CD14-positive in MO cultured in HT-29 conditioned medium without FCS supplemented with 2% human AB serum for seven days; F: No down-regulation of CD14 expression was observed on histogram of CD14 expressing mononuclear cells after seven days of culture in RPMI (control medium), J82 (control cell line) or WiDR conditioned medium; G: Histogram of CD14 expressing mononuclear cells after seven days of culture in DMEM (control medium) and HT-29 conditioned medium.
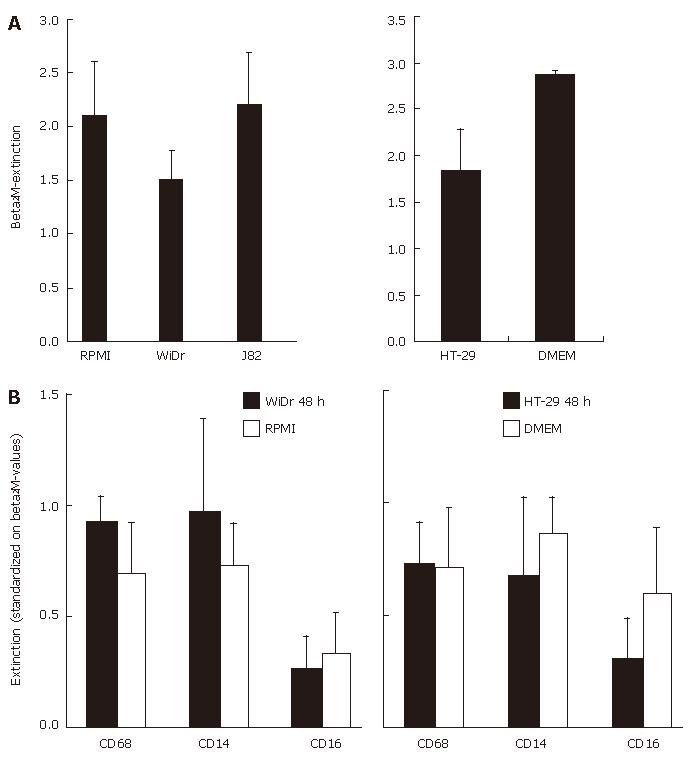
Figure 3 Cell ELISA of MO/MAC antigen expression after seven days of co-culture with IEC conditioned media.
A: Freshly elutriated MO were incubated in conditioned medium of the IEC line WiDr or control media (left graph) of HT-29 and control media (right graph) for seven days. Expression of the housekeeping antigen β2M was determined an extinction is given as absolute value; B: CD68, CD14 and CD16 antigen expression of MO incubated in conditioned medium of the IEC line WiDr or non-conditioned control medium (left graph) or HT-29 and control medium (right graph) after seven days was also determined by cell ELISA. Values are standardized on β2M-extinction.
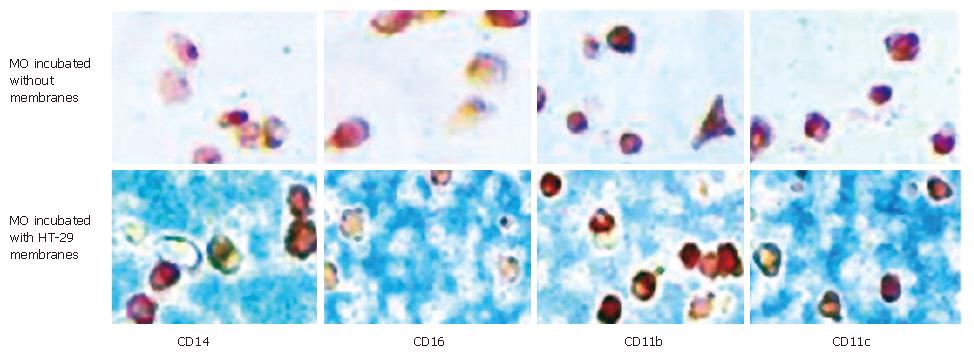
Figure 4 MO/MAC antigen expression after seven days of culture with IEC membranes.
Freshly elutriated MO were incubated for seven days with membrane preparations of the IEC line HT-29 or without membranes. Antigen expression was determined by immunohistochemistry (APAAP-method). There was no difference in expression of CD14, CD16, CD11b and CD11c in cells incubated with or without IEC membranes.
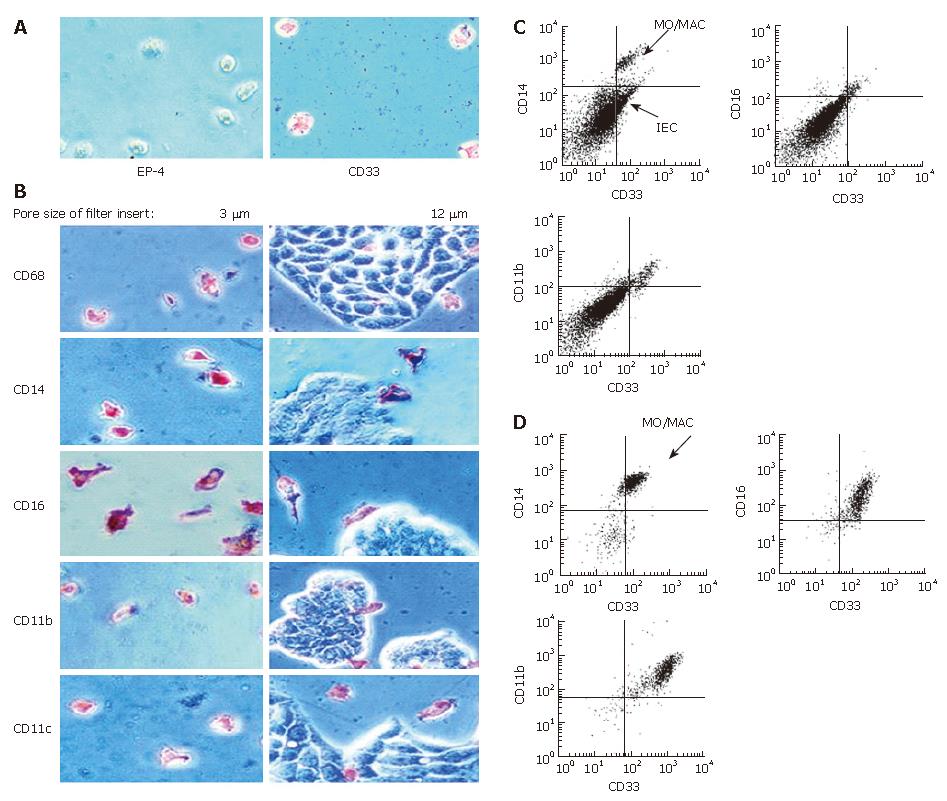
Figure 5 Antigen expression of MO/MAC incubated with IEC in trans-well cultures.
Freshly elutriated MO were incubated with the IEC line HT-29 seeded on filter inlays for seven days. Depending on pore size of the inlays only MO or MO and IEC could migrate through the filter. Antigen expression of migrating and non-migrating cells was examined by immunohistochemistry (APAAP-method) and flow cytometry. A: Migrating cells adhered to the plastic surface of the cell culture plate. When filters with a pore size of 3 μm were used, only MO were able to migrate through the membrane. Migrating cells were all negative for the epithelial cell specific marker EP-4 and positive for the MO/MAC-marker CD33; B: Migrating cells showed expression of CD68, CD14, CD16, CD11b and CD11c (left column). Twelve μm pores allowed migration of MO and IEC. None of the tested antigens was expressed by IEC (right column); C: Non migrating cells which remained in the upper compartment of the filter insert were examined by flow cytometry. The CD33-positive cell population (MO/MAC) showed also expression of CD14, CD16 and CD11b; D: Migrating cells which did not adhere to the plastic dish were examined by flow cytometry. Cells showed CD33-expression and were positive for CD14, CD16 and CD11b.
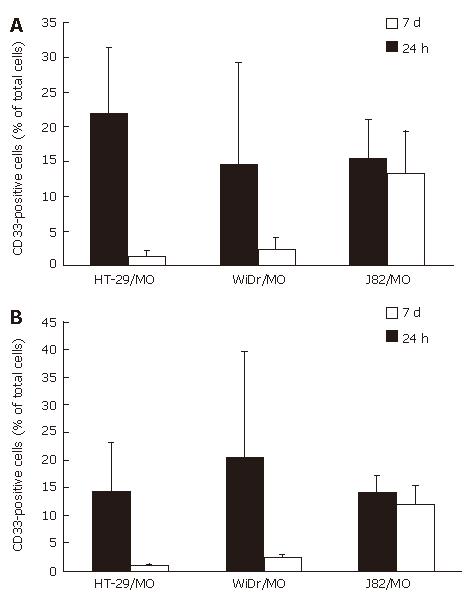
Figure 6 Mixed spheroids of MO and IEC or the control cell line.
Mixed spheroids of the IEC lines HT-29 and WiDr or the control cell line J82 and MO were generated and cultured for seven days, disaggregated and examined by flow cytometry. A: There was a strong decrease of surviving MO/MAC (CD33+ cells) inside IEC spheroids compared to control spheroids over the seven-day culture period. In HT-29 spheroids the percentage decreased from 21.7% (24 h) to 1.4% (7 d). In WiDr spheroids a slighter decrease was observed with 14.6% MO/MAC after 24 h and 2.4% after seven days. In spheroids of the control cell line J82 no selection of a MO/MAC subpopulation could be observed. The number of MO/MAC inside the aggregates was nearly constant with 15.2% (24 h) and 13.2 (7 d); B: Addition of a blocking anti-Fas antibody 30 min before generation of the mixed spheroids did not change the results. In IEC-MCS a strong decrease of MO/MAC was observed (HT-29: 14.2% 24 h, 0.8% 7 d, WiDr: 20.1% 24 h, 2.3% 7 d). The MO/MAC number in control cell MCS was nearly constant with 13.9% (24 h) and 12.0% (7 d).
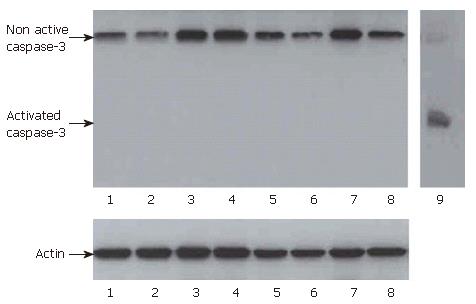
Figure 7 Western-blot for caspase-3 of mixed spheroids after 24 h and three days.
Mixed spheroids of IEC and MO, control cells and MO or control spheroids generated only from IEC or control cells were disaggregated after 24 h and three days of culture. Western-blots for activated caspase-3 were performed. Lane 1: HT-29 without MO 24 h; lane 2: HT-29/MO (1:1) 24 h; lane 3: J82 without MO 24 h; lane 4: J82/MO (1:1) 24 h; lane 5: HT-29 without MO 3 d; lane 6: HT-29/MO (1:1) 3 d; lane 7: J82 without MO 3 d; lane 8: J82/MO (1:1) 3 d; lane 9: positive control for activated caspase-3. No activated caspase-3 could be detected in co-cultures of MO and IEC or in spheroids generated only from IEC.
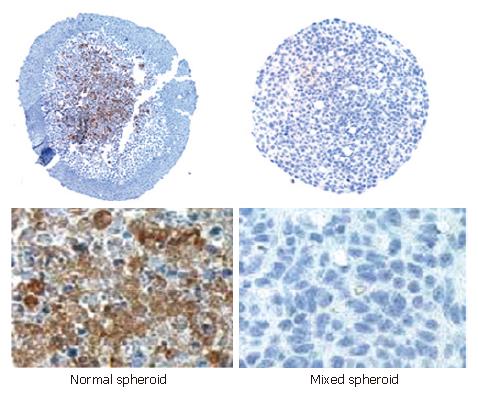
Figure 8 Immunohistochemical staining for the ECM-protein fibronectin in “normal” and mixed spheroids of the IEC line HT-29 after seven days.
Fibronectin could be detected in spheroids invaded by MO and cultured for seven days. Expression was preferentially localized in the center of aggregates. In mixed spheroids cultured for seven days no fibronectin expression was observed.
-
Citation: Spoettl T, Hausmann M, Menzel K, Piberger H, Herfarth H, Schoelmerich J, Bataille F, Rogler G. Role of soluble factors and three-dimensional culture in
in vitro differentiation of intestinal macrophages. World J Gastroenterol 2007; 13(7): 1032-1041 - URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1032.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1032