©2007 Baishideng Publishing Group Inc.
World J Gastroenterol. Jul 14, 2007; 13(26): 3540-3553
Published online Jul 14, 2007. doi: 10.3748/wjg.v13.i26.3540
Published online Jul 14, 2007. doi: 10.3748/wjg.v13.i26.3540
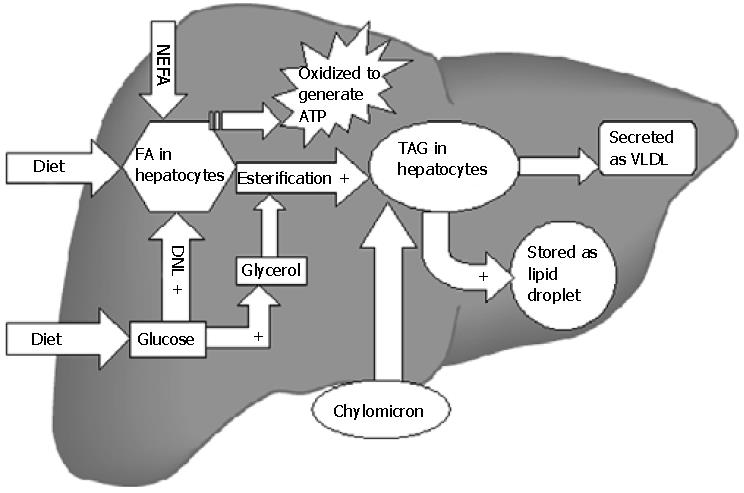
Figure 1 Lipid metabolism in liver.
All steps indicated by + are stimulated by insulin. Insulin suppresses the secretion of VLDL and the β-oxidation of fatty acids. Thus hyperinsulinemia in the setting of insulin resistance favors TAG accumulation in liver. TAG: triglycerides; VLDL: very low density lipoprotein; FA: fatty acid; NEFA: nonesterified fatty acids; DNL: de novo lipogenesis; ATP: adenosine triphosphate.
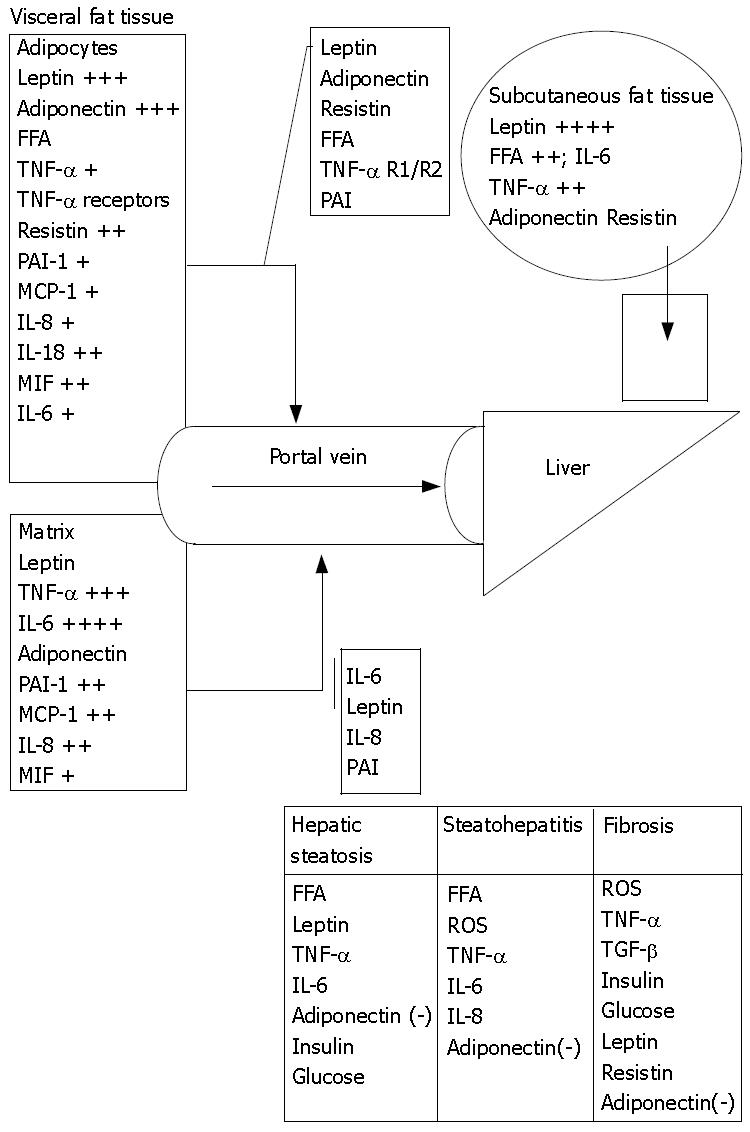
Figure 2 Portal/Visceral hypothesis.
Human adipose tissue (AT) is a potent source of inflammatory cytokines and that the majority of this release is due to the nonfat cells in the AT except for leptin and adiponectin that are primarily secreted by adipocytes. Adipocytes secrete at least as much PAI-1 (plasminogen activator inhibitor-1), MCP-1 (macrophage chemotaxis protein), IL-8 (Interleukin), and IL-6 in vitro as they do leptin but the nonfat cells of AT secrete even more of these proteins. The secretion of leptin by the nonfat cells is negligible. Obesity markedly elevates the total release of TNF-α, IL-6, and IL-8 by AT. Visceral fat releases more resistin, IL-6, PAI-1, TGF β1, IL-8 and IL-10 per gram of tissue than subcutaneous fat. (+) indicates fold increase in secretion in obesity; (-) indicates protective effect.
- Citation: Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol 2007; 13(26): 3540-3553
- URL: https://www.wjgnet.com/1007-9327/full/v13/i26/3540.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i26.3540