©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Jan 7, 2007; 13(1): 104-124
Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.104
Published online Jan 7, 2007. doi: 10.3748/wjg.v13.i1.104
Figure 1 Eastern wood-chuck (Marmota mo-nax).
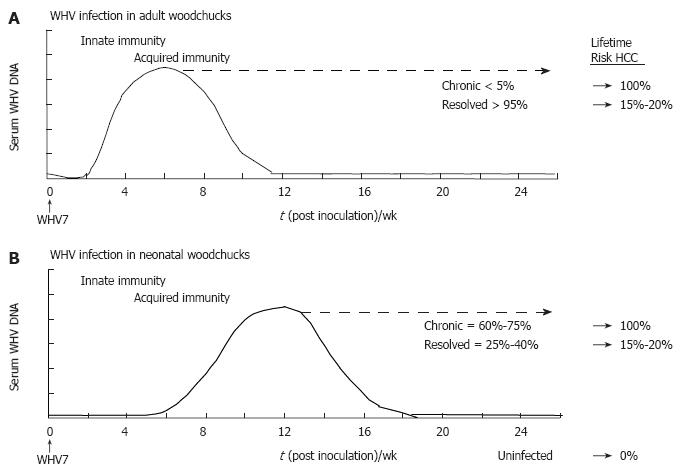
Figure 2 Schematic profiles for serum viremia in adult and neonatal models of experimental WHV infection.
A: Adult woodchucks. Adult woodchucks born to WHV-negative dams are infected with 1 x 107 woodchuck infectious doses 50% of a defined WHV inoculum by the intravenous route. The proportions of chronic and resolved outcomes of adult woodchucks usually are less than 5% and more than 95%, respectively. The lifetime risk for the development of HCC in established chronic and resolved WHV infections is 100% and 15%-20%, respectively; B: Neonatal woodchucks. Neonatal woodchucks born to WHV-negative dams are infected with 5 x 106 woodchuck infectious doses 50% of a defined WHV inoculum by the subcutaneous route. The proportions of chronic and resolved outcomes range between 60%-75% and 25%-40%, respectively. The lifetime risk for the development of HCC in established chronic and resolved WHV infections is 100% and 15%-20%, respectively. HCC in uninfected, WHV-negative woodchucks is not observed. Approximate time intervals for the development of innate and acquired immunity are shown.
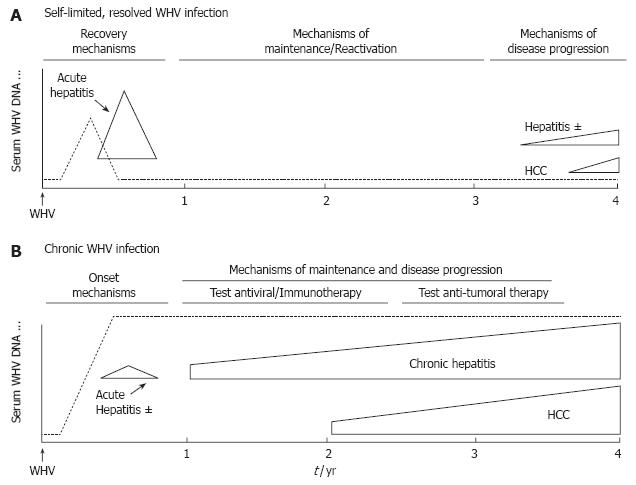
Figure 3 Schematic profiles for viremia, acute hepatitis, and disease progression in the neonatal model of experimental WHV infection.
A: Self-limited, resolved WHV infection; B: Chronic WHV infection. Neonatal woodchucks born to WHV-negative dams are infected experimentally at 3 d of age with 5 x 106 woodchuck infectious doses 50% of a defined WHV inoculum by the subcutaneous route. Approximate time intervals for self-limited acute hepatitis and progressive chronic hepatitis are shown. Use of the neonatal WHV infection model enables co-temporal comparisons of acute self-limiting and chronic outcome of WHV infections as they develop. Comparison of acute phase events at early time points before or near the times when self-limited, resolved and chronic outcomes begin to segregate based on serologic criteria allows the definition of immune mechanisms involved in progression toward recovery versus chronicity. Comparison at later time points enables the investigation of mechanisms that are important in the maintenance of the established WHV infection state and that lead to disease progression and tumor development. During this time the established chronic WHV carrier woodchucks are used mainly for the testing of antiviral nucleosides, immunotherapeutic strategies, and for the prevention of onset and development of HCC.
Figure 4 Combination treatment with clevudine and WHsAg vaccine suppresses serum viremia and antigenemia and induces a humoral response in chronic WHV carrier woodchucks.
Changes in serum WHV DNA, serum WHsAg, and anti-WHs antibodies of chronic carriers in response to treatment with placebo (CLV-V-), vaccine (CLV-V+), drug (CLV+V-), and combination of drug and vaccine (CLV+V+) are shown. Horizontal bars denote the period of clevudine (CLV) administration for 32 wk. Arrowheads represent the 4 immunizations (V) using 50 μg doses of an alum-adsorbed, formalin-inactivated WHsAg vaccine at wk 32, 36, 40, and 48. WHVge, WHV genomic equivalents (virion or WHV DNA-containing particles).
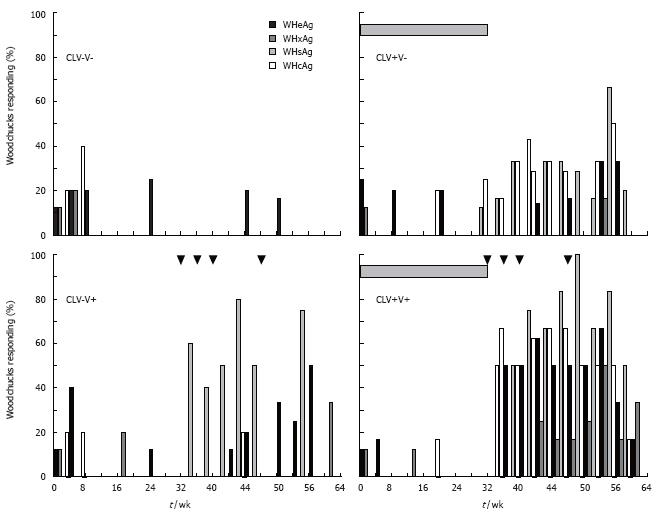
Figure 5 Combination treatment with clevudine and WHsAg vaccine enhances and expands the pattern of cell-mediated immune responses to WHV antigens in chronic WHV carrier woodchucks.
Changes in the PBMC responses to WHsAg, WHcAg, WHeAg, and WHxAg of chronic carriers in response to treatment with placebo (CLV-V-), vaccine (CLV-V+), drug (CLV+V-), and combination of drug and vaccine (CLV+V+) are shown. Horizontal bars denote the period of clevudine (CLV) administration for 32 wk. Arrowheads represent the 4 immunizations (V) using 50 μg doses of an alum-adsorbed, formalin-inactivated WHsAg vaccine at wk 32, 36, 40, and 48.
- Citation: Menne S, Cote PJ. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J Gastroenterol 2007; 13(1): 104-124
- URL: https://www.wjgnet.com/1007-9327/full/v13/i1/104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i1.104