©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 14, 2006; 12(46): 7497-7502
Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7497
Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7497
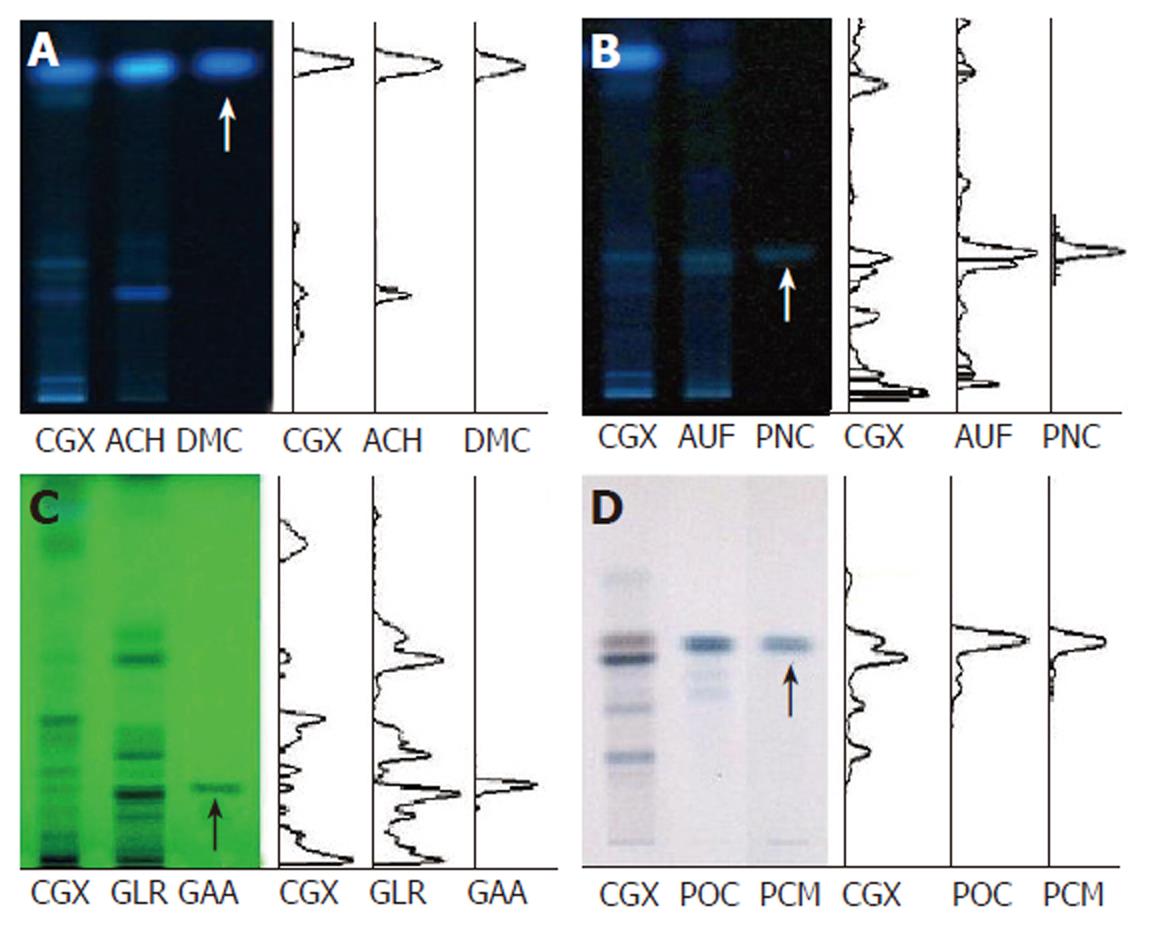
Figure 1 High-performance thin layer chromatography method-based fingerprint for Chunggan extract.
HPTLC analysis was performed to characterize CGX and its three major components (extracts from Artemisia capillaris Herba, Aurantii Fructus, Glycyrrhizae Radix and Poria cocos) with reference components, 6,7-dimethoxycoumarin, Poncirin, Glycyrrhizic acid ammonium salt and Pachyman (indicated by arrows). A: 2 μL of CGX (200 mg/mL) and 1 μL of the extract from Artemisia capillaris Herba (50 mg/mL) were subjected to HPTLC with 1 μL of 6,7-dimethoxycoumarin (0.1 mg/mL), then visualized under UV radiation at 366 nm; B: 2 μL of CGX (200 mg/mL) and 1 μL of the extract from Aurantii Fructus (50 mg/mL) were subjected to HPTLC with 1 μL of Poncirin (5 mg/mL), then visualized under UV radiation at 366 nm; C: 4 μL of CGX (200 mg/mL) and 4 μL of the extract from Glycyrrhizae Radix (50 mg/mL) were subjected to HPTLC with 4 μL of Glycyrrhizic acid ammonium salt (1 mg/mL), then visualized under UV radiation at 254 nm; D: 2 μL of CGX (40 mg/mL) and 1 μL of the extract from Poria cocos (10 mg/mL) were subjected to HPTLC with 10 μL of Pachyman (100 mg/mL), then visualized under white light after derivatization.
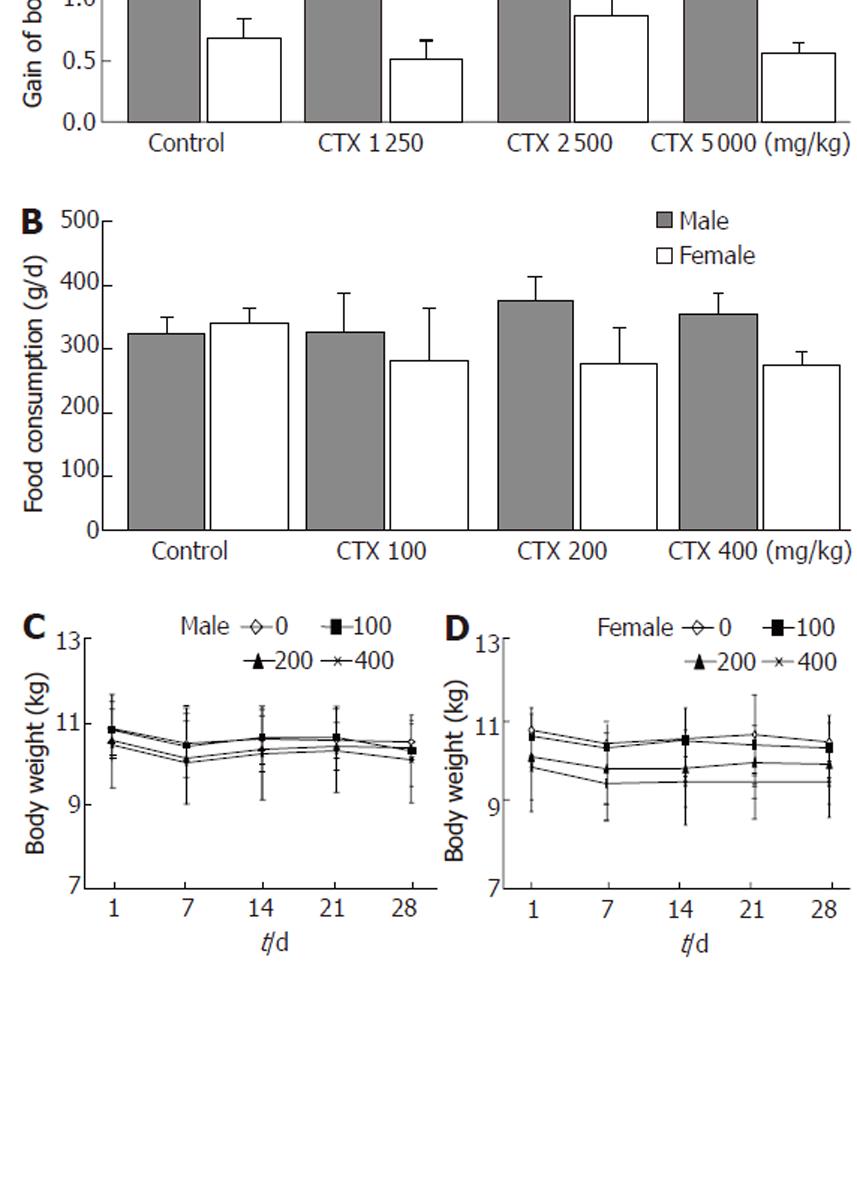
Figure 2 Body weight and food consumption.
A: Final gains in body weight for each group administered with indicated Chunggan extract (CGX) after 2 wk of the acute toxicity experiment (n = 2 males and 2 females); B: Average daily food consumption for each group (n = 3 males and 3 females) during the subacute toxicity experiment; C: Change in body weight during 4 wk of the subacute toxicological experiment, shown separately for males (n = 3) and females (n = 3). Values represent the mean ± SD.
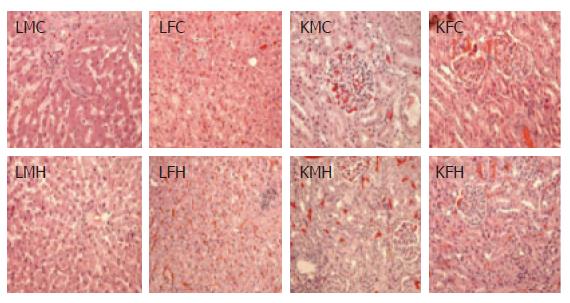
Figure 3 Histopathological findings.
On the final day of the subacute toxicological experiment, 13 main organs (indicated in the text) were removed from each dog, and microscopic examinations were carefully performed after a general H&E staining process. Livers, lungs, kidneys, spleens, and stomachs from the control and the group fed CGX revealed uniformly rare focal pathological findings. This figure presents the only pathological findings among livers and kidneys from the control and the group treated with the highest level of CGX, such as focal necrosis and hyperemia in livers, and glomerular hyperemia in kidneys (HE stain, X 200). LMC: liver from male control; LFC: liver from female control; LMH: liver from male high dose (400 mg/kg); LFH: liver from female high dose (400 mg/kg); KMC: kidney from male control; KFC: kidney from female control; KMH: kidney from male high dose (400 mg/kg); KFH: kidney from female high dose (400 mg/kg).
- Citation: Choi WJ, Shin JW, Son JY, Seo DS, Park HS, Han SH, Sung HJ, Cho JH, Cho CK, Yoo HS, Lee YW, Son CG. Toxicological study of the hepatotherapeutic herbal formula, Chunggan extract, in beagle dogs. World J Gastroenterol 2006; 12(46): 7497-7502
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7497