Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Jul 21, 2006; 12(27): 4281-4295
Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4281
Published online Jul 21, 2006. doi: 10.3748/wjg.v12.i27.4281
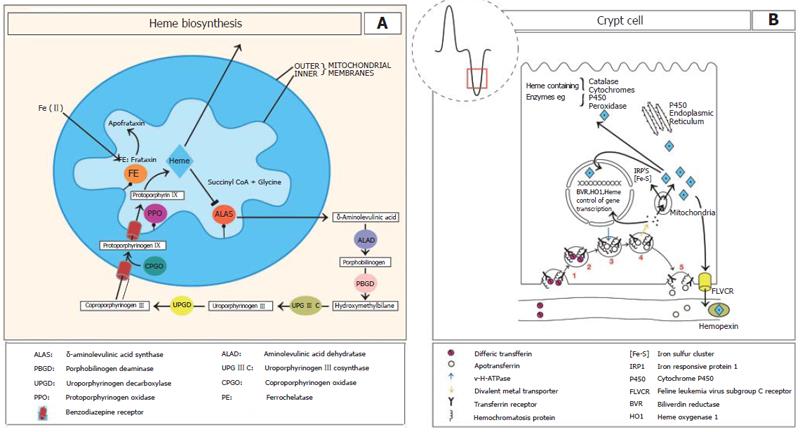
Figure 1 A: The heme biosynthetic pathway.
Mitochondrial and cytosolic locations of the eight enzymes are shown circled and coloured. Commencing synthesis is ALAS on the inner mitochondrial membrane of the first intermediate as well as subsequent intermediates. Heme synthesis is regulated by heme at the level of ALAS via feedback repression. It has been suggested that frataxin may donate ferrous iron to protoporphyrin in the formation of heme; B: In the intestinal crypts the uptake of plasma transferrin-iron occurs by the transferrin receptor (TfR). In iron deficiency HFE complexes with TfR1 and to a much lesser extent with iron loading. (1) TfR binds to plasma diferric transferrin. (2) TfR is internalised by receptor mediated endocytosis. (3) In the cytoplasm a v-H+ATPase fuses with the endosome and acidifies it to release the iron from transferrin. Following ferrireduction Fe(II) is transported to the cytoplasm by a metal transporter. (4) possibly divalent metal transporter 1 (DMT1). The iron is then transported into the mitochondria where it is incorporated into heme. The mitochondria are also a major producer of iron sulphur clusters. (5) The transferrin receptor - apotransferrin complex then return to the cell membrane where at the neutral pH, apotransferrin dissociates. Heme, heme oxygenase and BVR may regulate gene transcription during enterocyte differentiation. FLVCR functions to export excess heme.
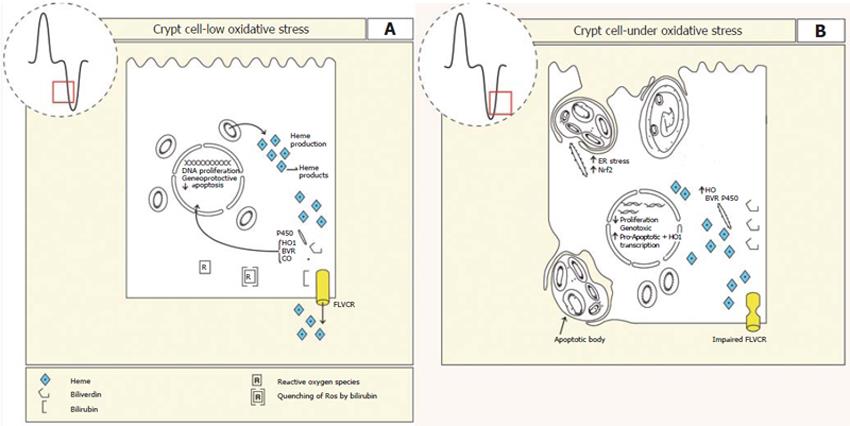
Figure 2 A: Epithelium of the crypt region is active in cell proliferation and differentiation.
Heme production is required for the synthesis of heme containing enzymes. In these cells there are also high levels of heme oxygenase activity suggesting that heme breakdown is required for the production of bilirubin and carbon monoxide to maintain appropriate proliferation, differentiation and apoptosis. If the oxygen tension of the cell should increase or production of heme exceeds use, as would be seen as differentiation proceeds, then excess heme may be exported via FLVCR to limit oxidative stress. Increased oxidative stress may also be buffered by the antioxidant bilirubin; B: In the presence of increased oxidative stress caused by excess heme production, impaired FLVCR transport or increased oxygen tension, heme increases to levels that are genotoxic and the cell is predisposed to pro-apototic gene expression placing the cell into a death programme. Normal intestinal growth and differentiation would be impaired.
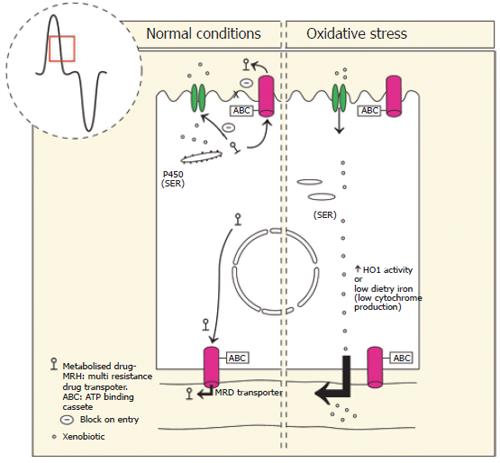
Figure 3 Left: Xenobiotics in the diet enter the enterocyte via facilitated diffusion or a specific transport process.
Appropriate P450 expression on smooth endoplasmic reticulum (SER) enables first pass metabolism including phase I, and phase II metabolizing enzymes. Phase III multi drug resistance transporters (MDR) transport the conjugated-xenobiotic compound to the lumen or blood stream where increased hydrophilicity impairs re-entry into the enterocyte and leads to its elimination from the body directly. De novo synthesis of P450 occurs in the enterocytes and is dependent on appropriate levels of dietary iron. Right: In the presence of oxidative stress caused by high dietary intake of metals or compounds that induce heme oxygenase 1 (HO-1), heme containing P450 are broken down leading to increased entry of xenobiotics to the body. Dietary iron deficiency leads to reduced P450 activity and reduced detoxification capabilities.
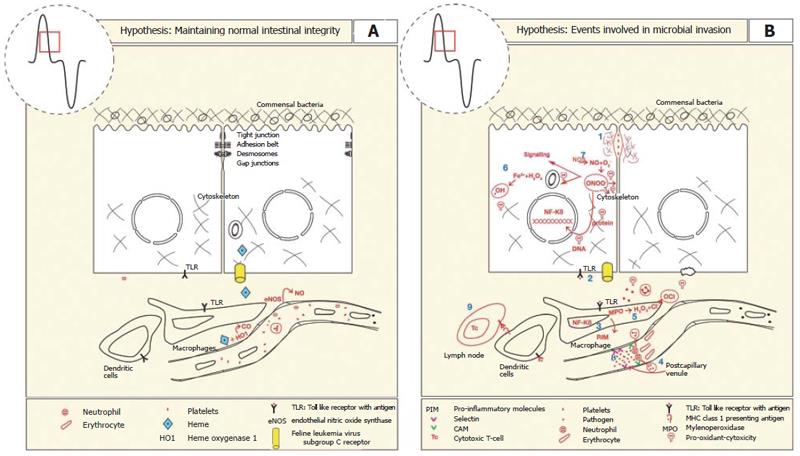
Figure 4 A: The intestinal mucosal barrier is maintained by a series of lateral membrane specialisations near the apical pole of the epithelial cell.
It comprises tight junctions, adhesion belts, desmosomes and gap junctions that prevent the movement of pathogens across the epithelial monolayer. Constitutive expression and activities of endothelial nitric oxide synthase (eNOS) and heme oxygenase 1 (HO-1) is important for maintaining adequate blood flow, anti-inflammatory, anti-thromobotic (-) and anti-apoptotic effects on endothelium, neutrophils, platelets, and enterocytes, respectively. HO-1 activity produces the antioxidant bilirubin to limit oxidative damage; B: The loss of mucosal integrity results in the translocation of pathogens and establishment of an inflammatory response by the following series of events. (1) Initiation of synthesis of proteases by bacteria erode tight junction complexes between epithelial cells. (2) Binding of bacterial motifs activates toll like receptors, initiating the NF-kB pathway. (3) Increased expression of pro-inflammatory cytokines, chemokines and endothelial cell surface adhesion molecules. (4) Leukocytes extravasate and increased permeability of capillaries increases fluid accumulation (5) Phagocytic cells produce myeloperoxidase which combines with peroxide to form hypochlorous acid that damages pathogen and host systems alike. (6) Peroxide produced by enterocytes in combination with ferrous iron can produce superoxide anions that damage lipids, DNA and proteins. (7) NO is produced at high concentrations that combines with peroxide to form the pro-oxidant peroxynitrite. (8) Platelets also bind to the endothelial surface to induce hemostasis. (9) Presentation of antigens by dendritic cells via major histocompatibility class 1 to cytotoxic T-cells leads to antibody presentation and destruction of infected epithelial cells.
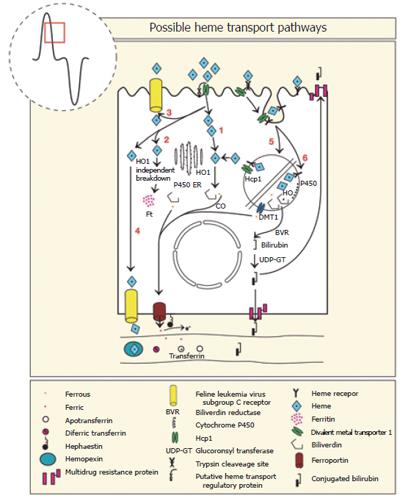
Figure 5 Six steps in the uptake of heme by intestinal enterocytes.
Heme taken up by heme carrier protein (HCP1) is internalised and broken down in the cytoplasm by HO-1 (1), by a HO-1 independent enzyme(s) (2), some is released intact back into the lumen (3) or plasma by FLVCR (4). Heme may also bind to a heme receptor and with HCP1 be internalised by receptor mediated endocytosis. The heme may be released to the cytoplasm by HCP1 (5), or the heme may be broken down in the lysosome and the released iron transported to the cytoplasm by the divalent metal transporter (DMT1) (6). The iron released from heme passes to the basal cytoplasm and is transported across the basal membrane by ferroportin in the ferrous state, oxidized to ferric-iron by hephaestin and transported in the plasma by transferrin.
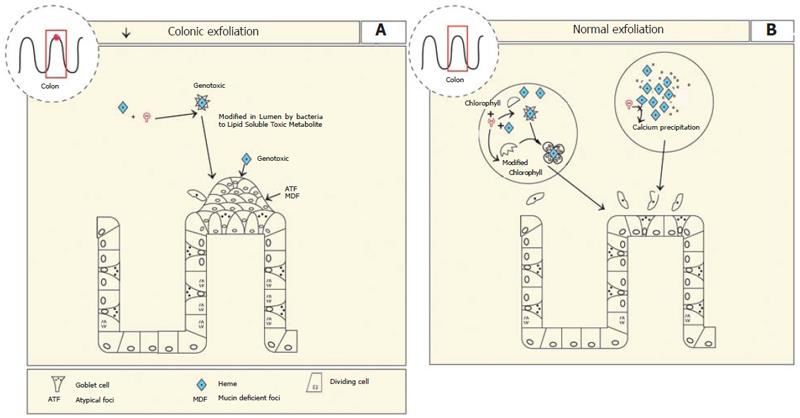
Figure 6 A: In the colon excess heme is metabolised into a lipid soluble heme metabolite possibly by commensal bacteria.
Heme itself is also genotoxic. This results in the formation of aberrant atypical foci, that are mucin deficient (ATF, MDF). Apoptosis is inhibited which could lead to increased survival of mutant cells; B: In the presence of calcium or chlorophyll heme precipitates into biological inactive compounds which inhibit the heme factor or binds the heme factor rendering it inert, respectively leading to normal colon growth.
- Citation: Oates PS, West AR. Heme in intestinal epithelial cell turnover, differentiation, detoxification, inflammation, carcinogenesis, absorption and motility. World J Gastroenterol 2006; 12(27): 4281-4295
- URL: https://www.wjgnet.com/1007-9327/full/v12/i27/4281.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i27.4281