©The Author(s) 2005.
World J Gastroenterol. Oct 14, 2005; 11(38): 5966-5972
Published online Oct 14, 2005. doi: 10.3748/wjg.v11.i38.5966
Published online Oct 14, 2005. doi: 10.3748/wjg.v11.i38.5966
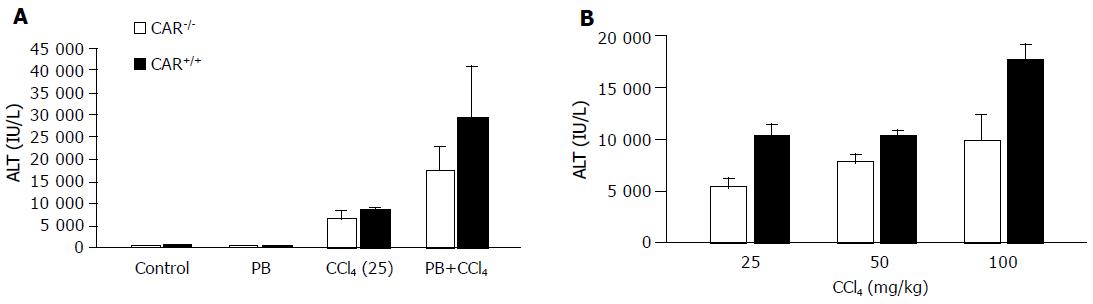
Figure 1 (A) PB pretreatment induced CCl4 toxicity.
CAR+/+ or CAR–/– mice were administered a 25-mg/kg dose of CCl4 by intraperitoneal injection with or without PB pretreatment (n=6 per treatment group). Serum was collected and ALT levels were measured after 24 h. The ALT level was slightly higher in CAR+/+ mice than in CAR–/– mice with CCl4 treatment (P<0.001). The difference in ALT levels became clearer between CAR+/+ and CAR–/– mice with pre-treatment by PB (P<0.001). (B) Dose dependency of CCl4 toxicity. CAR+/+ and CAR–/– mice were given 25-, 50-, or 100-mg/kg doses of CCl4. Blood samples were collected 24 h later, and serum ALT levels were measured (n=4). CAR–/– animals were significantly less sensitive than CAR+/+ mice to CCl4 toxicity (P<0.001). Data are mean±SD.
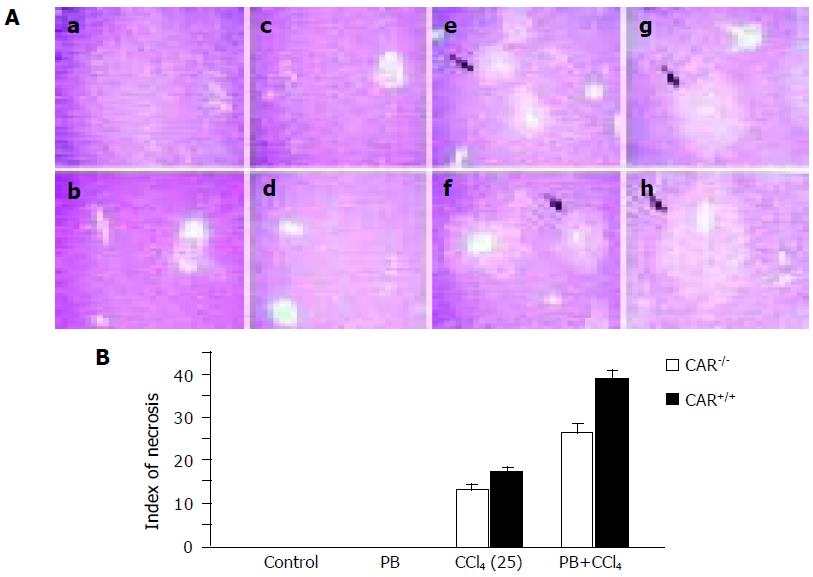
Figure 2 Histological changes associated with CCl4 toxicity.
(A) Histological findings in the liver; (a) CAR–/– mice, control; (b) CAR+/+ mice, control; (c) CAR–/– mice, PB; (d) CAR+/+ mice, PB; (e) CAR–/– mice, CCl4; (f) CAR+/+ mice, CCl4; (g) CAR–/–mice, PB plus CCl4; and (h) CAR+/+ mice, PB plus CCl4. Liver sections from each treatment were examined by hematoxylin and eosin staining. Liver samples from all treated animals were analyzed, but only representative histology is presented. The extent of the necrotic area in the CAR+/+ liver was larger than that in CAR–/– mice. PB pretreatment caused extensive centrilobular necrosis in CAR+/+ mice. The arrows indicate centrilobular necrosis. Magnification, ×100. (B) The indices of centrilobular necrosis in CAR+/+ mice and CAR–/– mice. The indices of centrilobular necrosis were higher in CAR+/+ mice than that in CAR–/– mice with PB pretreatment. The index in CAR+/+ mice treated with CCl4 plus PB was significantly increased. The index of centrilobular necrosis was scored as follows: area of centrilobular necrosis divided by whole area. Data are mean±SD.
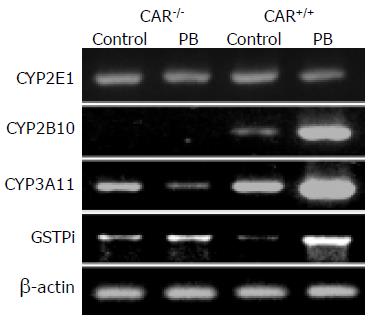
Figure 3 Hepatic mRNA level of drug-metabolizing enzymes with PB treatment.
Total liver RNA was prepared from CAR+/+ or CAR-/- animals treated with a 100-mg/kg dose of PB by intraperitoneal injection (n = 6 per treatment group). Total liver RNA was prepared 12 h after PB treatment and subjected to PCR analysis with the indicated primers in Materials and methods. b-Actin mRNA level was also measured as an internal control. The amplified DNA was separated on a 1.5% agarose gel and visualized with staining by ethidium bromide. The expected sizes of the amplified cDNA are described in Materials and methods.
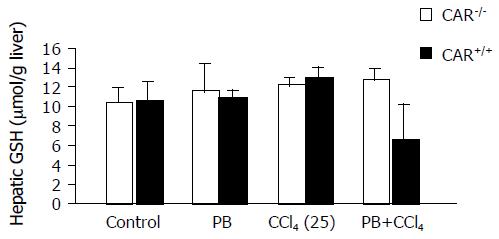
Figure 4 Hepatic glutathione (GSH) level.
Liver samples treated with the indicated chemicals were collected 24 h after each treatment and glutathione levels were measured (n = 6 per treatment group) using the total glutathione quantification kit. Hepatic glutathione levels in the PB plus CCl4 treated CAR+/+ mice significantly decreased, compared with those of the CAR-/- mice (P<0.001). Data are mean±SD.
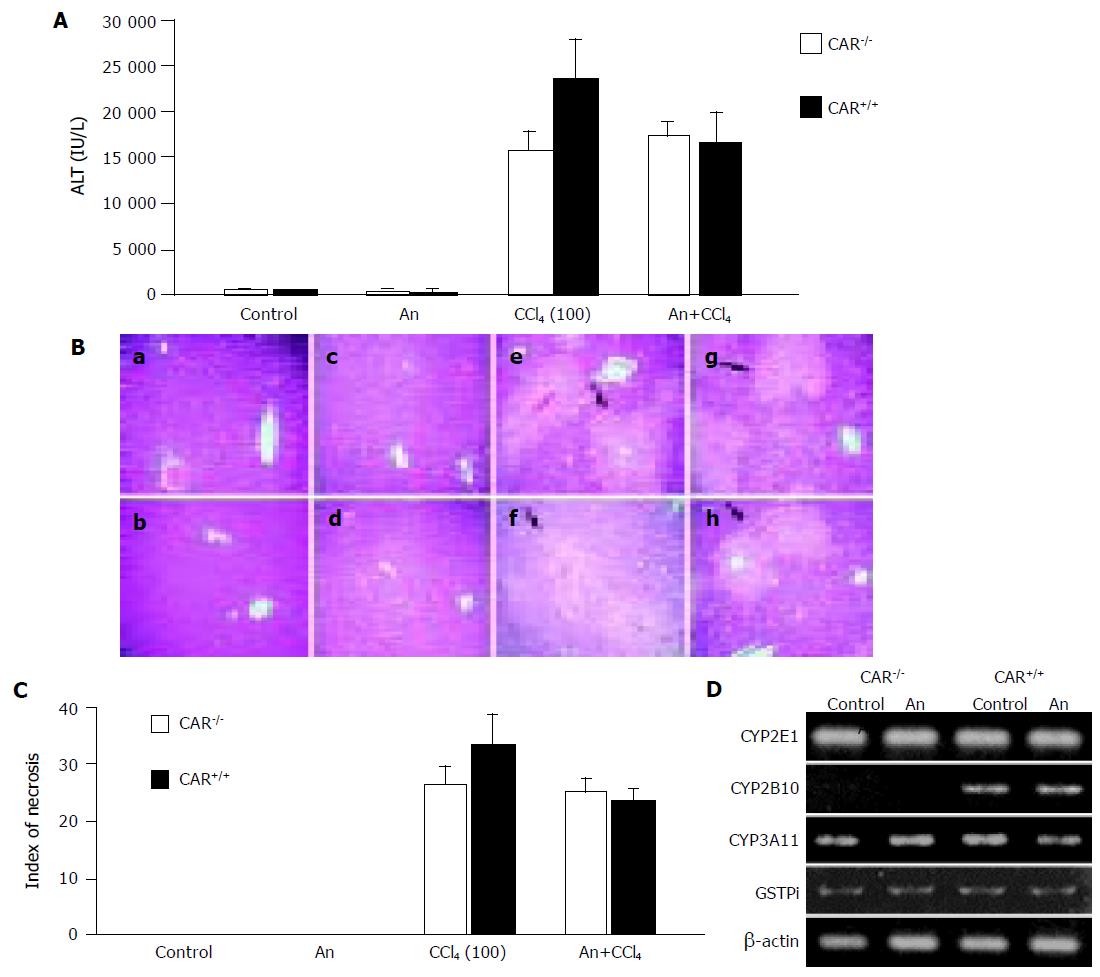
Figure 5 Androstanol prevented CCl4-induced hepatotoxicity.
(A) CAR+/+ or CAR–/– mice were given a 100-mg/kg dose of CCl4 by intraperitoneal injection, with or without pretreatment with androstanol (100 mg/kg). Serum ALT levels were measured 24 h later (n=6 per treatment group). Androstanol-pretreatment reduced the ALT level of CAR+/+ mice to the level of CAR–/– mice. An: androstanol. (B) Liver sections from the same animals 24 h after different treatments, as indicated, were stained with hematoxylin and eosin. (a) CAR–/– mice, control; (b) CAR+/+ mice, control; (c) CAR–/– mice, androstanol; (d) CAR+/+ mice, androstanol; (e) CAR–/– mice, CCl4; (f) CAR+/+ mice, CCl4; (g) CAR–/– mice, androstanol plus CCl4; and (h) CAR+/+ mice, androstanol plus CCl4. Androstanol pretreatment reduced the hepatic centrilobular necrosis in CAR+/+ mice. Arrows indicate areas of hepatic necrosis. (C) The indices of centrilobular necrosis in CAR+/+ mice and CAR–/– mice. Androstanol-pretreatment reduced hepatic centrilobular necrosis of CAR+/+ mice to the level of CAR–/– mice. Data in (A) and (C) are mean±SD. (D) Hepatic mRNA level of drug-metabolizing enzymes with androstanol treatment. Total liver RNA was prepared from CAR+/+ or CAR–/– animals treated with a 100-mg/kg dose of androstanol by intraperitoneal injection (n=6 per treatment group) and subjected to PCR analysis with the indicated primers in Materials and methods. β-Actin mRNA level was also measured as an internal control. The amplified DNA was separated on a 1.5% agarose gel and visualized with staining by ethidium bromide. The expected sizes of the amplified cDNA are described in Materials and methods.
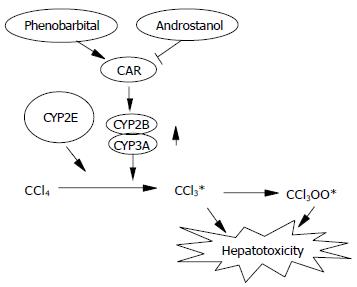
Figure 6 Schematic representation of CAR-mediated CCl4 hepatotoxicity.
- Citation: Yamazaki Y, Kakizaki S, Horiguchi N, Takagi H, Mori M, Negishi M. Role of nuclear receptor CAR in carbon tetrachloride-induced hepatotoxicity. World J Gastroenterol 2005; 11(38): 5966-5972
- URL: https://www.wjgnet.com/1007-9327/full/v11/i38/5966.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i38.5966