©The Author(s) 2005.
World J Gastroenterol. Jul 14, 2005; 11(26): 4024-4031
Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4024
Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4024
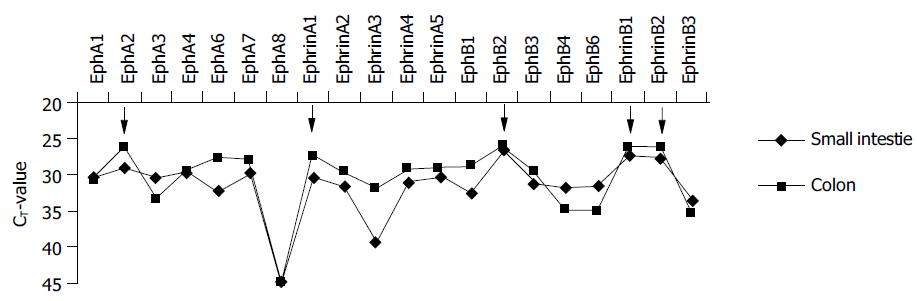
Figure 1 Expression profile of Eph-RTKs and ephrins in normal human mucosa of the small intestine (n = 5) and colon (n = 5) assessed by real time TaqMan® RT-PCR.
The CT-values allow a relative quantification of the mRNA expression of the single members. Low CT-values indicate a high expression level and vice versa. The arrows indicate marked expression of the family members EphA2, ephrin-A1, EphB2, and ephrin-B1/2.
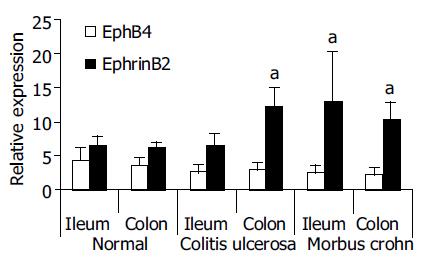
Figure 2 Differential gene expression of Eph-RTKs and ephrins in the mucosa of IBD patients (TaqMan® RT-PCR validation of Affymetrix® cDNA array results[17]).
Individual RNAs from perilesional total mucosa biopsies (ileum terminale and colon) of 4 control subjects, 4 MC patients and 4 CU patients were analyzed. Real-time RT-PCR confirmed significant up-regulation of ephrin-B2 in MC patients (ileum and colon) and CU (colon). Differential expression of EphB4, as suggested by the Affymetrix® array data, was not significant. The relative expression values were calculated according to the standard curve method of the TaqMan® protocol (Applied Biosystems, Darmstadt, Germany) by normalization with a housekeeper gene (18S rRNA) and are therefore unitless (for details, see[15]). aP < 0.05 vs normal.
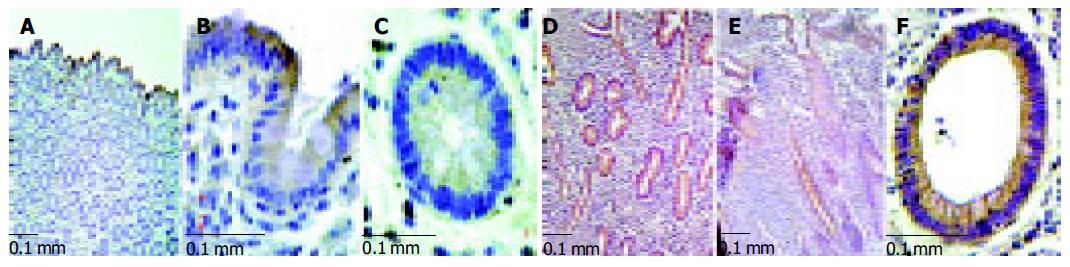
Figure 3 Immunohistochemistry of ephrin-B2 from biopsies of normal colon and lesional colon in IBD.
(A-C: normal colon; D, F: MC; E: CU.). A-C: ephrin-B2 expression shows a physiologic gradient along the crypt-axis in normal epithelium of the colon, i.e. the maximum staining is found in the luminal, terminally differentiated epithelium of normal colon mucosa. D-F: In MC and CU this gradient is disturbed, i.e. strong ectopic expression in the deep parts of the crypts can be observed. C and F represent cross sections of the deep part of a crypt.
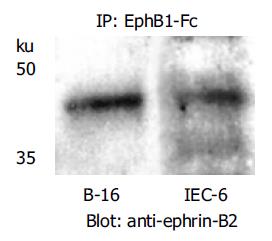
Figure 4 Immunoprecipitation of ephrin-B2 with EphB1-Fc demonstrates that the recombinant rat EphB1-Fc binds to ephrin-B2 (42 ku), and ephrin-B2 is constitutively expressed by rat IEC-6 cells.
Ephrin-B2 transfected B16 melanoma cells served as positive control.
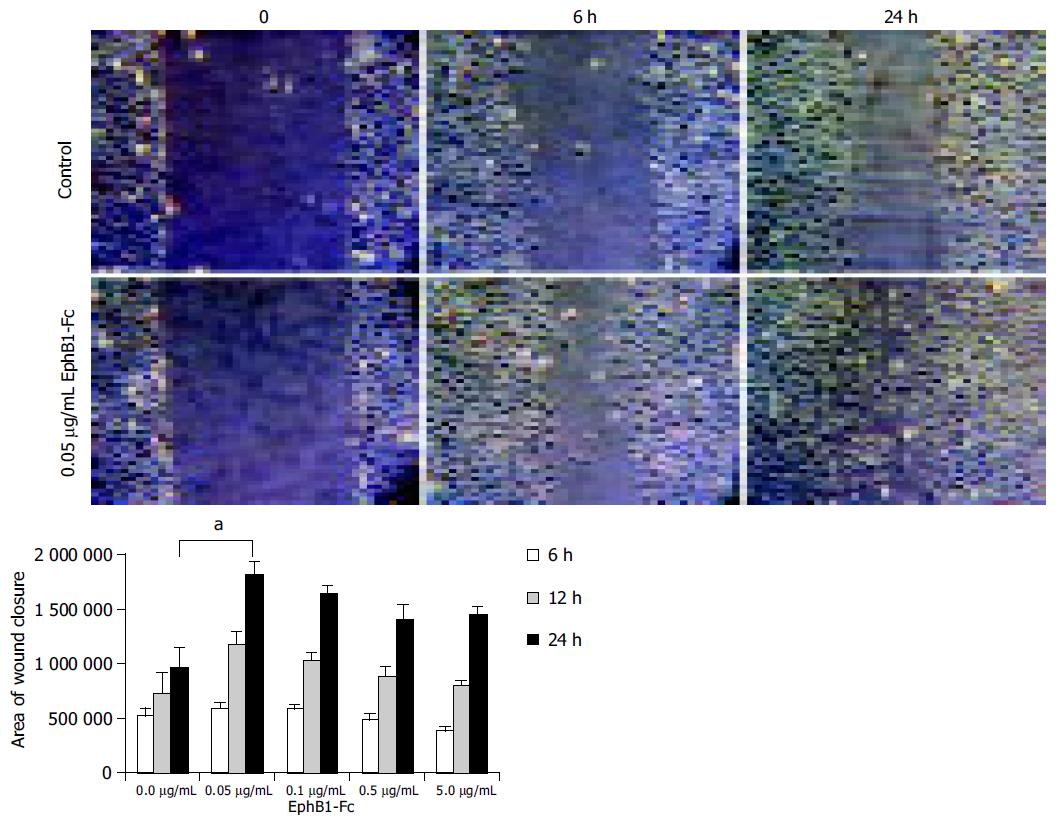
Figure 5 IEC-6 in vitro scratch wound assays indicate an enhanced wound closure activity upon stimulation with EphB1-Fc.
IEC-6 cell monolayers were wounded in a standardized manner using a cell scraper, and relative wound closure was monitored after 6 h and 24 h. Subnanomolar doses of EphB1-Fc (0.05 µg/mL~0.33 nmol/L) significantly enhanced the wound closure (aP < 0.05). Higher doses did not further increase this effect.
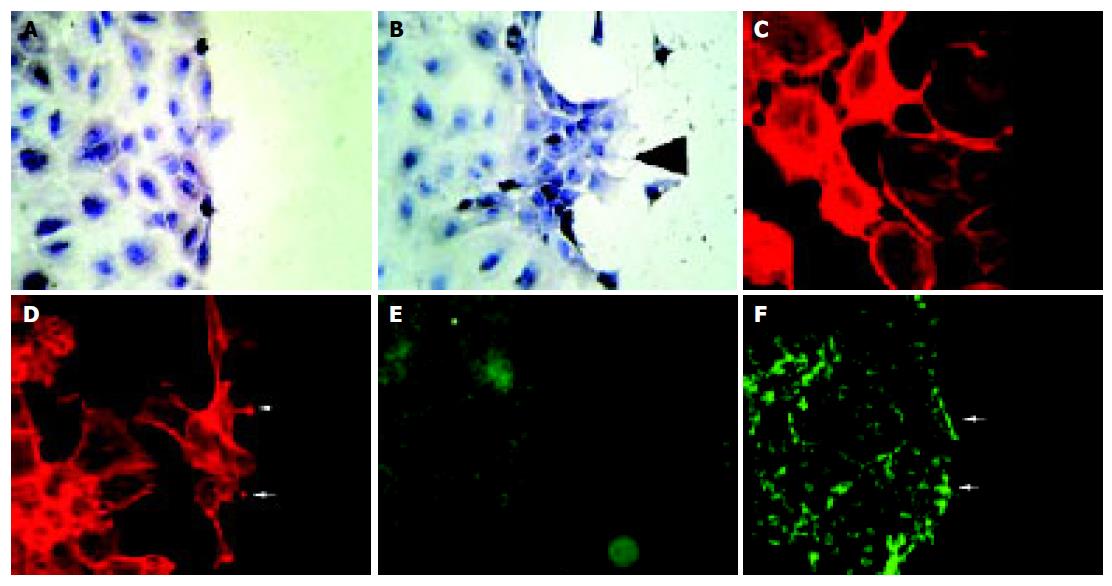
Figure 6 Stimulation of IEC-6 cells with EphB1-Fc induces subcellular responses compatible with enhanced wound closure capacity.
Compared to the non-stimulated control (A), recombinant EphB1-Fc stimulated cells produce an organized migratory activity forming multiple archipelago-like sheets of cells stretching into the denuded area already 6 h after stimulation. In addition, asymmetric lamellipodia are seen (arrow). (B). Staining of actin with phalloidin shows a rounded morphology with less polymerized actin bundles in unstimulated cells (C), whereas EphB1-Fc stimulated cells display a stretched-out, bizarre morphology and apparent assembly of abundant stress fibers. Spike-like protrusions may correspond to focal adhesion assembly (arrows) (D). EphB1-Fc also induces an increased deposition of laminin as a provisional basal membrane at the wound edge (F) (arrows) compared to control cells (E).
- Citation: Hafner C, Meyer S, Langmann T, Schmitz G, Bataille F, Hagen I, Becker B, Roesch A, Rogler G, Landthaler M, Vogt T. Ephrin-B2 is differentially expressed in the intestinal epithelium in Crohn’s disease and contributes to accelerated epithelial wound healing in vitro. World J Gastroenterol 2005; 11(26): 4024-4031
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/4024.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.4024