©2005 Baishideng Publishing Group Inc.
World J Gastroenterol. Apr 28, 2005; 11(16): 2371-2384
Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2371
Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2371
Math 1 Math(A1).
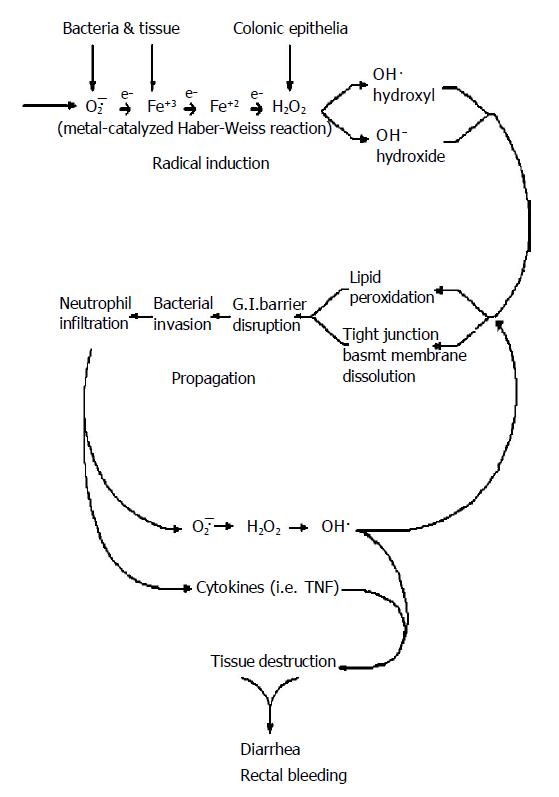
Figure 1 Pathogenesis of UC: Individuals with UC are basically normal.
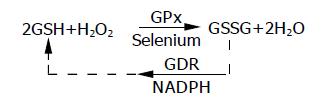
Polymorphic genes (MTHFR and PGD) and significant inter-individual variability in anti-oxidant capacity places certain individuals at the lower threshold of their physiological hydrogen peroxide reducing capability for any given environmental oxidant stress level. Oxygen radical production is induced by environmental oxidant stressors (xenobiotics, stress, smoking cessation, hypermetabolic states) interacting with cellular metabolism. This process is called “Radical Induction”. Excess un-neutralized hydrogen peroxide generated during the radical induction phase diffuses from intracellular compartments of colonic epithelial cells, through the plasma membrane, to the extracellular space. Free extracellular hydrogen peroxide reacts with superoxide (O2·) in a transition metal catalyzed Haber-Weiss reaction to form hydroxyl radical (HO·) and hydroxide (HO—) as follows: *O2—·+Fe+3 (or Cu)→O2+Fe+2 (followed by) Fe+2+H2O2→Fe+3+ HO—+HO·. Hydroxyl radical initiates oxidative damage to structures that comprise the gastrointestinal barrier (epithelial TJs, BM and epithelial lipid peroxidation) resulting in transient immune activation, which ceases when the damage is repaired. Intermittent immune activation by colonic bacterial antigens can lead to antibody formation (panca) and extra-intestinal manifestations. When oxidative damage to the GI barrier cannot be repaired in time to prevent subjacent endothelial adhesion molecule expression, WBCs (neutrophils) begin infiltrating into the damaged colonic epithelium in an effort to prevent systemic bacteremia, which would otherwise result in fatal sepsis. Resident mucosal neutrophils produce additional large quantities of diffusible H2O2 which causes oxidative tissue damage in adjacent colonic epithelial cells whose anti-oxidant (GSH) levels have already been previously compromised by radical induction. This results in a proximal “advancing edge” of oxidative tissue damage, TJ disruption and lipid peroxidation extending proximally from the rectum, a unique tissue with high oxidant exposure secondary to fecal generated oxygen radicals, maximum bacterial antigenic load and least anti-oxidant defense as compared to the rest of the GI tract. The accompanying intense neutrophilic diapedesis in a restricted area of epithelial TJ disruption is followed initially by microscopic erythrocyte extravasation eventually leading to frank hemorrhage as endothelial junctions fail to close leading to bloody diarrhea and further neutrophil infiltration characteristic of this disease. The process is only temporarily halted when sufficient anti-oxidant (GSH) capacity is encountered producing a clear line of demarcation between diseased and normal tissue. This self-perpetuating and auto-stimulating cycle is called propagation. *Both iron (Fe) and superoxide (O2—) are plentiful within the colonic lumen[108-110]. UC has been reported subsequent to oral iron supplementation for the treatment of anemia[111] and in association with conditions of copper overload (i.e., Wilson’s disease)[112].
- Citation: Pravda J. Radical induction theory of ulcerative colitis. World J Gastroenterol 2005; 11(16): 2371-2384
- URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2371.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2371