©2005 Baishideng Publishing Group Inc.
World J Gastroenterol. Jan 7, 2005; 11(1): 114-117
Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.114
Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.114
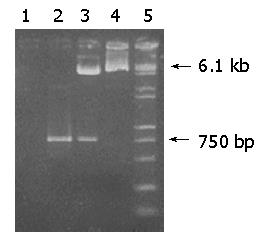
Figure 1 Agarose gel electrophoresis analysis of recombinant pIRES-hpaA.
Lane 1: PCR product of pIRES as a negative control; lane 2: PCR product of pIRES-hpaA; lane 3: pIRES-hpaA after digestion with EcoR I and Mlu I; lane 4: pIRES after digestion with Eco RI and Mlu I; lane 5: DNA Marker (DL2000+15000).
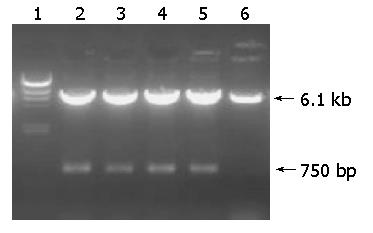
Figure 2 Agarose gel electrophoresis analysis of recombinant attenuated Salmonella typhimurium DNA vaccine strain with restriction enzyme digestion.
Lane 1: DNA ladder (1 kb); lanes 2-5: Recombinant plasmid pIRES-hpaA from strains of different generations after digestion with EcoRI and Mlu I; lane 6: pIRES after digestion with EcoRI and Mlu I.
Figure 3 Identification of recombinant attenuated Salmonella typhimurium DNA vaccine strain carrying hpaA by PCR.
Lane 1: Product amplified from pIRES as a negative control; lane 2: Marker (DL2000+15000); lanes 3-4: Products amplified from recombinant plasmid from strains of different generations by PCR.
Figure 4 Western blotting of expressed pIRES-hpaA products.
Lane 1: COS-7 cells transfected by pIRES as a control; Lane 2: COS-7 cells transfected by pIRES-hpaA.
-
Citation: Xu C, Li ZS, Du YQ, Tu ZX, Gong YF, Jin J, Wu HY, Xu GM. Construction of a recombinant attenuated
Salmonella typhimurium DNA vaccine carryingHelicobacter pylori hpaA. World J Gastroenterol 2005; 11(1): 114-117 - URL: https://www.wjgnet.com/1007-9327/full/v11/i1/114.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.114