©The Author(s) 2004.
World J Gastroenterol. Aug 15, 2004; 10(16): 2379-2382
Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2379
Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2379
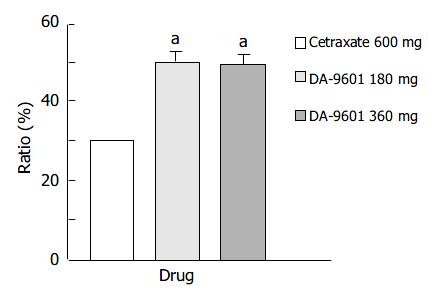
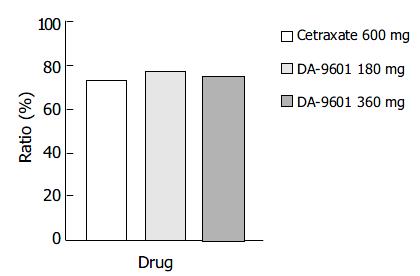
Figure 1 Estimated cure rates of erosive gastritis by intention-to-treat (ITT) analysis among patients treated with DA-9601 (180 mg or 360 mg, t.
i.d.) or cetraxate (600 mg, t.i.d.) for 2 wk. aP < 0.05 vs 600 mg of cetraxate.
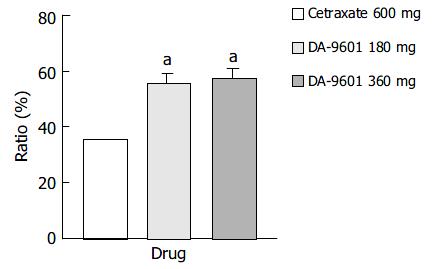
Figure 2 Estimated cure rates of erosive gastritis by per proto-col (PP) analysis among patients treated with DA-9601 (180 mg or 360 mg, t.
i.d.) or cetraxate (600 mg, t.i.d.) for 2 wk. aP < 0.05 vs 600 mg of cetraxate.
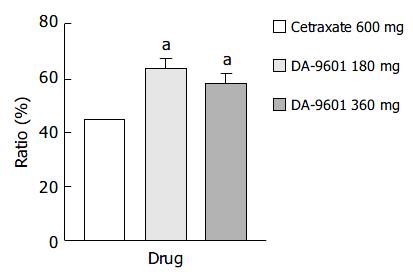
Figure 3 Estimated improvement rates of erosive gastritis by intention-to-treat (ITT) analysis among patients treated with DA-9601 (180 mg or 360 mg, t.
i.d.) or cetraxate (600 mg, t.i.d.) for 2 wk. aP < 0.05 vs 600 mg of cetraxate.
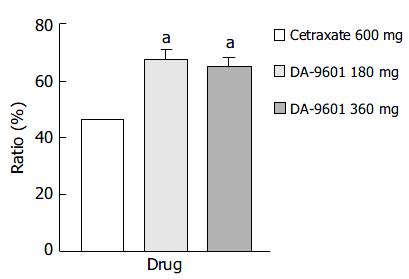
Figure 4 Estimated improvement rates of erosive gastritis by per protocol (PP) analysis among patients treated with DA-9601 (180 mg or 360 mg, t.
i.d.) or cetraxate (600 mg, t.i.d.) for 2 wk. aP < 0.05 vs. 600 mg of cetraxate.
Figure 5 Overall reduction rates of symptoms among patients treated with cetraxate (600 mg, t.
i.d.) or DA-9601 (180 mg and 360 mg, t.i.d.) for 2 wk.
- Citation: Seol SY, Kim MH, Ryu JS, Choi MG, Shin DW, Ahn BO. DA-9601 for erosive gastritis: Results of a double-blind placebo-controlled phase III clinical trial. World J Gastroenterol 2004; 10(16): 2379-2382
- URL: https://www.wjgnet.com/1007-9327/full/v10/i16/2379.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i16.2379