Published online Feb 28, 2022. doi: 10.35711/aimi.v3.i1.1
Peer-review started: December 13, 2021
First decision: January 26, 2022
Revised: February 14, 2022
Accepted: February 17, 2022
Article in press: February 17, 2022
Published online: February 28, 2022
Processing time: 77 Days and 1.9 Hours
Artificial intelligence (AI) is a branch of computer science where machines are trained to imitate human-level intelligence and perform well-defined tasks. AI can provide accurate results as well as analyze vast amounts of data that cannot be analyzed via conventional statistical methods. AI has been utilized in pulmonary medicine for almost two decades and its utilization continues to expand. AI can help in making diagnoses and predicting outcomes in pulmonary diseases based on clinical data, chest imaging, lung pathology, and pulmonary function testing. AI-based applications enable physicians to use enormous amounts of data and improve their precision in the treatment of pulmonary diseases. Given the growing role of AI in pulmonary medicine, it is important for practitioners caring for patients with pulmonary diseases to understand how AI can work in order to implement it into clinical practices and improve patient care. The goal of this mini-review is to discuss the use of AI in pulmonary medicine and imaging in cases of obstructive lung disease, interstitial lung disease, infections, nodules, and lung cancer.
Core Tip: Artificial Intelligence (AI) has the potential to have a tremendous influence when dealing with pulmonary diseases. This review provides a glimpse of AI application in pulmonary medicine and explains how AI uses imaging data to facilitate precision medicine in our data-driven era.
- Citation: Choudhury S, Chohan A, Dadhwal R, Vakil AP, Franco R, Taweesedt PT. Applications of artificial intelligence in common pulmonary diseases. Artif Intell Med Imaging 2022; 3(1): 1-7
- URL: https://www.wjgnet.com/2644-3260/full/v3/i1/1.htm
- DOI: https://dx.doi.org/10.35711/aimi.v3.i1.1
Artificial Intelligence (AI) is a branch of computer science that aims to imitate human thinking ability, learning, planning, and reasoning to solve complex problems. In 1956, scientists began theorizing a computer's ability to learn new information by analyzing data which led to the beginning of the field of AI[1]. While the terms AI, machine learning and deep learning are often used similarly, the relationship between them needs to be clarified to avoid confusion. AI is the overall concept of the simulation of human intelligence using computer systems[2]. Meanwhile, machine learning (ML) is a field of AI which provides knowledge or information using its capability of learning and analyzing massive amounts of data from larger datasets including more variables than conventional statistical methods. Machine learning uses various algorithms to process data, such as supervised learning, unsupervised learning and reinforced learning[1]. Supervised learning involves the computer recognizing patterns from data using guidance. Whereas, unsupervised learning involves pattern recognition by the computer without any guidance[2]. Reinforced learning has the ability to recognize and analyze data without any labels, by using incremental positive or negative feedback[3]. Deep learning is a subset of ML that enables the algorithm to learn from a training data set and apply that to fulfill intended tasks to a new data set[2]. As healthcare data has become increasingly complex, AI has the potential to have a significant influence on medical data analysis and medical practice.
AI has been implemented in many fields of medicine to facilitate precision medicine by predicting outcomes, diagnosis, and therapeutic results. AI may assist in diagnosis of different diseases by recognizing the images from different parts of the body, predicting mortality in the critical care unit, classifying skin biopsies, and identifying new genotypes in heart failure. The US Food and Drug Administration (FDA) and Conformité Européenne (CE)-marked have approved more than 300 AI-based software/medical devices[4,6]. Many of them are related to pulmonary imaging (Table 1)[4,6].
| Pulmonary conditions | AI device/algorithm | Imaging | Brief description |
| Chronic obstructive pulmonary disease | Lung density analysis software | Chest CT | Uses three-dimensional segmentation of the lungs, volumetric analysis and density evaluations from CT images to aid in diagnosis and progression of the disease |
| LungQ software | Chest CT | Quantitative analysis of lung volume. Airway morphology analysis | |
| Interstitial lung disease | LungPrint Discovery | Chest CT | Lung tissue and airway evaluation. Quantitative analysis using deep learning to detect interstitial lung disease and chronic obstructive lung disease |
| Lung Texture Analysis | Chest CT | Transforms a standard chest CT into a detailed map. Lung textures quantification | |
| Pulmonary infection | Icolung | Non-contrast Chest CT | Detects COVID-19 at an early stage and quantify the extent of lung lesions |
| InferRead CT pneumonia | Chest CT | Real-time identification. Alerts of suspected pneumonia cases | |
| Lung nodule | Syngo.CT Lung CAD | Multidetector Chest CT | Computer-aid detection tool designed to detect solid pulmonary nodules using convolutional neural network. To be used as the second reader. |
| AI-Rad Companion (Pulmonary) | CT DICOM chest | Quantitative and qualitative analysis using deep learning. Segmentation of lung lobes and identification of lesions | |
| Temporal Comparison software | Chest X-ray | The new image is superimposed on the old image to detect changes in the lung parenchyma. | |
| ClearRead CT | CT chest | Lung nodule detection asymptomatic population |
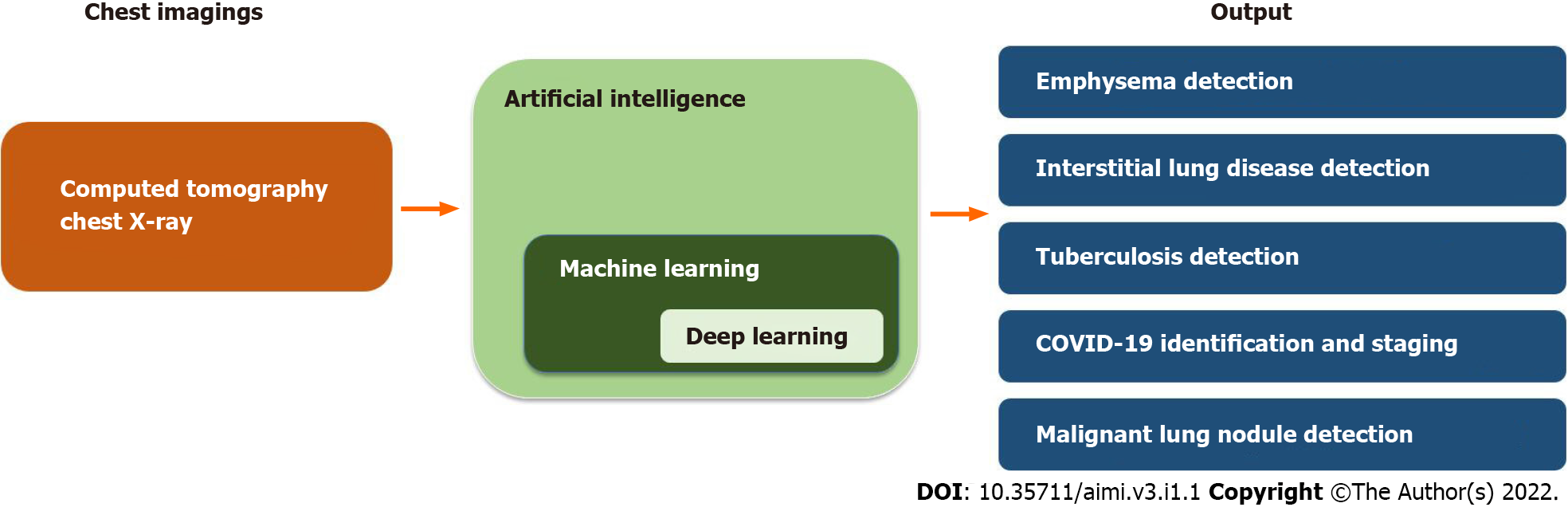
In the 1980s, AI was initially introduced into pulmonary medicine to interpret lung function tests[5]. Since then, AI has been applied in various pulmonary diseases, including, but not limited to obstructive lung diseases, pulmonary infections, interstitial lung disease, and malignancy[6]. Given its widespread use in pulmonary medicine, it is important for pulmonologists to have a general understanding of the utilization of AI in this field and how it can aid them in caring for patients. In this narrative mini-review, we provided an overview of the pulmonary diseases that are commonly diagnosed and managed by general pulmonologists for which AI has been applied including obstructive lung disease, interstitial lung disease, pulmonary tuberculosis (TB), coronavirus disease 2019 (COVID-19) pneumonia, lung nodules and lung cancer (Figure 1.).
PubMed was searched from inception to November 30, 2021, using keywords: “artificial intelligence, lung disease”, “ artificial intelligence, pulmonary disease”, “artificial intelligence, COPD, asthma”, “ artificial intelligence, interstitial lung disease”, “artificial intelligence, tuberculosis”, “artificial intelligence, COVID-19”, and “artificial intelligence, lung nodule, lung cancer”. All types of published publications were included, e.g., reviews, observational studies, and meta-analyses. We prioritized recent articles within five years in this narrative mini-review.
The gold standard of diagnosis in obstructive lung diseases like asthma and chronic obstructive pulmonary disease (COPD) involves a combination of signs, symptoms, and spirometry. While AI cannot replace the clinicians’ role, it can complement clinicians’ interpretation of the data available at the bedside. A study by Topalovic et al[7] compared the accuracy of pulmonologists’ interpretation of pulmonary function testing to an AI-based software that used more than 1430 historical patient cases. Both groups were asked to study 50 patient cases and correctly interpret the pulmonary function test while placing them in diagnostic categories. AI-based software was found to outperform the pulmonologist interpretation by a substantial margin[7].
According to the Global Strategy for Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) reports 2022, COPD is one of the top three causes of death in the world[8]. Moll et al[9] also proposed a machine learning mortality prediction model for patients with COPD based on six-minute walk tests, percent predicted of forced expiratory volume in 1 second (FEV1), and age. While the gold standard of diagnosis of COPD is spirometry, studies have suggested that artificial intelligence and deep learning can potentially be utilized to screen patients for COPD. Tang et al[10] suggests that low dose computed tomography (CT) screening of the lungs of both smokers and ex-smokers can be examined using deep residual networks to identify patients who may have COPD but remain undiagnosed. AI has also been used to characterize patients already diagnosed with COPD. The Genetic Epidemiology Study (COPDGene) is one of the largest data sets obtained over ten years, consisting of chest imaging, spirometry, and molecular data from patients with COPD. This has been used as the source for multiple studies that have related specific COPD phenotypes to genetic and molecular mechanisms and has led to the prediction of the disease progression of various COPD subtypes[11]. A study by Fischer et al[12] describes an algorithm that can perform lung lobe segmentation and emphysema quantification, which has been shown to correlate with different GOLD stages in patients with COPD per their spirometry data. Furthermore, AI-based applications have also been suggested to help patients identify if they may be having an exacerbation at home and when they should seek help from a medical professional[13]. This can promote patient responsibility and potentially save on resources, including emergency department visits.
Asthma is an intermittent and reversible obstructive lung disease with multiple phenotypes. AI may improve diagnosis, phenotype classification, prediction of asthma exacerbations and treatment response[1,15]. Multiple studies have shown good accuracy of ML-based algorithms in screening and diagnosis of asthma in adult patients[1]. In regards to phenotype classification, when using the machine learning approach as well as cluster analysis, the highest corticosteroid-responsiveness phenotype was identified in patients with low pulmonary function, high serum eosinophils, nasal polyps, and late-onset asthma[14]. The least corticosteroid-responsiveness phenotype was also found in young, obese females with early-onset asthma[14]. In another study, Qin et al[15] adopted deep learning algorithms-based high-resolution computed tomography (HRCT) chest images to assess small airway thickness with the aim of steroids response evaluation in asthma patients with small airway obstruction. Phenotype identification can help tailor asthma management and possibly improve outcomes.
Interstitial lung disease (ILD) is an umbrella term that encompasses all disease processes that can cause pleural/parenchymal inflammation and scarring. Deep learning algorithms can help with the diagnosis of ILD using HRCT chest images. In a case-control study by Walsh et al[16], a database of 1157 de-identified HRCT images showing evidence of diffuse fibrotic lung disease were classified using the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association (ATS/ERS/JRS/ALAT) idiopathic pulmonary fibrosis guidelines. These images were divided into multiple groups and separately read by a deep learning algorithm and 91 thoracic radiologists. Walsh et al[16] found that the algorithm outperformed thoracic radiologists’ interpretation of HRCT images with the median accuracy of 73.3% vs 70.7%, respectively. This study showed that deep learning algorithms could serve as a valuable tool in the diagnosis of ILD. Similarly, Choe et al[17] has revealed that deep learning increases the diagnostic accuracy of chronic hypersensitivity pneumonitis, cryptogenic organizing pneumonia, nonspecific interstitial pneumonia, and usual interstitial pneumonia patterns. Other studies have used AI algorithms to evaluate HRCT images of patients with interstitial pulmonary fibrosis and have successfully been able to quantify airway volumes and parenchymal lesions[17,18].
The utilization of AI has also been investigated in multiple pulmonary infections. Here, we briefly review the utilization of AI in pulmonary tuberculosis and COVID-19.
Tuberculosis (TB) remains a significant cause of mortality in many parts of the world. Due to the variable presentations of TB in chest radiography, diagnosis remains a challenge. The first conventional computer-aided diagnosis (CAD) was made in 2016 to aid in the detection of TB. Over the years, investigators have also developed multiple CAD algorithms that can detect various radiographic findings in TB, for example, cavitary and focal TB[19]. In addition to diagnosis, AI can be helpful in other aspects of TB care as well. AI has been suggested as an aid to review records, identify symptomatic patterns, surveillance, and factors that may contribute to the treatment and medication adherence failure in TB[20]. Doshi et al[21] describe innovative ways in which AI-based software can provide access to care and facilitate the management of TB patients worldwide.
In recent times, COVID-19 has taken the world by storm. Morbidity and mortality around the world have risen as treatment options for COVID-19 remain largely experimental. AI software has been developed to aid in the early diagnosis and prognostication of patients with COVID-19. In a retrospective, multi-center study by Li et al[22], a deep learning model, called COVID-19 detection neural network was developed to identify CT findings of COVID-19 infection and differentiate it from CT findings in community-acquired pneumonia. Another study developed a deep learning convolution neural network to effectively stage the severity of COVID-19 infection via scoring of various radiographic features[23]. This can help in early prognostication of the disease, which can lead to making early treatment decisions. Another study by Burdick et al[24] used ML algorithm to build a model which uses inputs of diastolic blood pressure, systolic blood pressure, heart rate, temperature, respiratory rate, oxygen saturation, white blood cell, platelet count, lactate, blood urea nitrogen, creatinine, and bilirubin to predict the need for mechanical ventilation. Furthermore, investigators have developed deep learning algorithms which help to identify protein structures and shapes. The data provided using this algorithm has been invaluable in the development of the COVID-19 vaccine[6].
Despite recent advances in the treatment of pulmonary malignancies, the World Health Organization considers them among the deadliest of all solid malignancies[25]. Early and accurate diagnosis remains paramount in improving patient outcomes. CAD systems use deep learning algorithms as an aid for radiologists to analyze CT images by lung segmentation and provide a more focused analysis that will allow nodule detection and classification. One such state-of-the-art algorithm implemented by Siemen Healthcare uses statistical finite element analysis or three-dimensional lung segmentation in adversarial neural network training[26]. A study by Chauvie et al[27] compared different machine learning algorithms and lung-RADs criteria and concluded that neural network algorithms enhanced the positive predictive value in chest digital tomosynthesis in lung cancer detection. One identified disadvantage of deep learning is that it does not provide uniform features for identifying malignant versus benign nodules. This problem has been addressed using a method called Radiomics[28]. Radiomics uses features from one image in order to provide data-characterization algorithms that helps to identify similar features in new data. This tool can help in finding characteristics of malignancies that can be otherwise missed by human experts. The combination of Radiomics and deep learning promises the ability to provide radiologists around the world an advantage in diagnosing pulmonary malignancies. Finally, a study by Afshar et al[29] has proposed a deep learning-based Radiomics model to predict the time-to-event outcome prediction, that utilizes raw images of CT and PET (Positron Emission Tomography) scans and can calculate the image-based risk of death or recurrence, for each patient.
Despite the promising outcomes of AI, small or unstructured databases and missing data may result in unsatisfactory AI quality. For example, in the diagnosis of lung nodules and lung malignancy, the software’s ability is usually compared to the ability of expert radiologists. However, since the ultimate goal is to diagnose malignancies and not just identify lung nodules, algorithms should be made to focus on identifying malignancies with a different reference standard[30]. Similarly, AI poses other limitations as well. For example, characteristics of CT imaging are being primarily used as an input for AI algorithm to diagnose early COVID-19 infection. However, it should be noted that while CT scan has high sensitivity it does not have very high specificity for COVID-19. So, diagnosing the disease based solely on CT images with the help of AI may be erroneous[31]. Therefore, while AI has many advantages, it is important to keep these limitations in mind. Finally, cooperation between physicians and AI researchers is needed to be able to develop well-structured AI applications that can be validated in real-world study before launching AI models into clinical fields.
The implementation of AI and machine learning algorithms is an evolving and relevant topic in pulmonary medicine. Human errors can occur in the medical field. It can be associated with missed, late, and incorrect diagnoses leading to health and economic burden. AI is an efficient tool that can be implemented to prevent this problem by aiding in the fast, accurate, and early diagnosis, prognostication, as well as treatment of pulmonary diseases. Nonetheless, the lack of knowledge and confidence in applying AI into practice may hinder the utilization of AI in the medical field. Moreover, well-performed AI algorithms require a large well quality database. Physician and AI algorithm developers should work closely to minimize these limitations. While AI alone cannot replace clinician expertise, it can add to the armamentarium and improve patient care and healthcare worldwide.
| 1. | Feng Y, Wang Y, Zeng C, Mao H. Artificial Intelligence and Machine Learning in Chronic Airway Diseases: Focus on Asthma and Chronic Obstructive Pulmonary Disease. Int J Med Sci. 2021;18:2871-2889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (6)] |
| 2. | Kaplan A, Cao H, FitzGerald JM, Iannotti N, Yang E, Kocks JWH, Kostikas K, Price D, Reddel HK, Tsiligianni I, Vogelmeier CF, Pfister P, Mastoridis P. Artificial Intelligence/Machine Learning in Respiratory Medicine and Potential Role in Asthma and COPD Diagnosis. J Allergy Clin Immunol Pract. 2021;9:2255-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (4)] |
| 3. | Ali I, Hart GR, Gunabushanam G, Liang Y, Muhammad W, Nartowt B, Kane M, Ma X, Deng J. Lung Nodule Detection via Deep Reinforcement Learning. Front Oncol. 2018;8:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 4. | The Medical Futurist. The Medical Futurist. Available from: https://medicalfuturist.com/fda-approved-ai-based-algorithms/. |
| 5. | Aikins JS, Kunz JC, Shortliffe EH, Fallat RJ. PUFF: an expert system for interpretation of pulmonary function data. Comput Biomed Res. 1983;16:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 67] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 6. | Khemasuwan D, Sorensen JS, Colt HG. Artificial intelligence in pulmonary medicine: computer vision, predictive model and COVID-19. Eur Respir Rev. 2020;29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 7. | Topalovic M, Das N, Burgel PR, Daenen M, Derom E, Haenebalcke C, Janssen R, Kerstjens HAM, Liistro G, Louis R, Ninane V, Pison C, Schlesser M, Vercauter P, Vogelmeier CF, Wouters E, Wynants J, Janssens W; Pulmonary Function Study Investigators; Pulmonary Function Study Investigators:. Artificial intelligence outperforms pulmonologists in the interpretation of pulmonary function tests. Eur Respir J. 2019;53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (2)] |
| 8. | GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf. Available from: https://goldcopd.org/wp-content/uploads/2021/11/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf. |
| 9. | Moll M, Qiao D, Regan EA, Hunninghake GM, Make BJ, Tal-Singer R, McGeachie MJ, Castaldi PJ, San Jose Estepar R, Washko GR, Wells JM, LaFon D, Strand M, Bowler RP, Han MK, Vestbo J, Celli B, Calverley P, Crapo J, Silverman EK, Hobbs BD, Cho MH. Machine Learning and Prediction of All-Cause Mortality in COPD. Chest. 2020;158:952-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (3)] |
| 10. | Tang LYW, Coxson HO, Lam S, Leipsic J, Tam RC, Sin DD. Towards large-scale case-finding: training and validation of residual networks for detection of chronic obstructive pulmonary disease using low-dose CT. Lancet Digit Health. 2020;2:e259-e267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 11. | Castaldi PJ, Boueiz A, Yun J, Estepar RSJ, Ross JC, Washko G, Cho MH, Hersh CP, Kinney GL, Young KA, Regan EA, Lynch DA, Criner GJ, Dy JG, Rennard SI, Casaburi R, Make BJ, Crapo J, Silverman EK, Hokanson JE; COPDGene Investigators. Machine Learning Characterization of COPD Subtypes: Insights From the COPDGene Study. Chest. 2020;157:1147-1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 12. | Fischer AM, Varga-Szemes A, Martin SS, Sperl JI, Sahbaee P, Neumann D, Gawlitza J, Henzler T, Johnson CM, Nance JW, Schoenberg SO, Schoepf UJ. Artificial Intelligence-based Fully Automated Per Lobe Segmentation and Emphysema-quantification Based on Chest Computed Tomography Compared With Global Initiative for Chronic Obstructive Lung Disease Severity of Smokers. J Thorac Imaging. 2020;35 Suppl 1:S28-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (5)] |
| 13. | Swaminathan S, Qirko K, Smith T, Corcoran E, Wysham NG, Bazaz G, Kappel G, Gerber AN. A machine learning approach to triaging patients with chronic obstructive pulmonary disease. PLoS One. 2017;12:e0188532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 14. | Wu W, Bang S, Bleecker ER, Castro M, Denlinger L, Erzurum SC, Fahy JV, Fitzpatrick AM, Gaston BM, Hastie AT, Israel E, Jarjour NN, Levy BD, Mauger DT, Meyers DA, Moore WC, Peters M, Phillips BR, Phipatanakul W, Sorkness RL, Wenzel SE. Multiview Cluster Analysis Identifies Variable Corticosteroid Response Phenotypes in Severe Asthma. Am J Respir Crit Care Med. 2019;199:1358-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (2)] |
| 15. | Qin Y, Wang J, Han Y, Lu L. Deep Learning Algorithms-Based CT Images in Glucocorticoid Therapy in Asthma Children with Small Airway Obstruction. J Healthc Eng. 2021;2021: 5317403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (2)] |
| 16. | Walsh SLF, Calandriello L, Silva M, Sverzellati N. Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: a case-cohort study. Lancet Respir Med. 2018;6:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 270] [Article Influence: 33.8] [Reference Citation Analysis (2)] |
| 17. | Choe J, Hwang HJ, Seo JB, Lee SM, Yun J, Kim MJ, Jeong J, Lee Y, Jin K, Park R, Kim J, Jeon H, Kim N, Yi J, Yu D, Kim B. Content-based Image Retrieval by Using Deep Learning for Interstitial Lung Disease Diagnosis with Chest CT. Radiology. 2022;302:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 18. | Handa T, Tanizawa K, Oguma T, Uozumi R, Watanabe K, Tanabe N, Niwamoto T, Shima H, Mori R, Nobashi TW, Sakamoto R, Kubo T, Kurosaki A, Kishi K, Nakamoto Y, Hirai T. Novel Artificial Intelligence-based Technology for Chest Computed Tomography Analysis of Idiopathic Pulmonary Fibrosis. Ann Am Thorac Soc. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (2)] |
| 19. | Kulkarni S, Jha S. Artificial Intelligence, Radiology, and Tuberculosis: A Review. Acad Radiol. 2020;27:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 20. | Chopra KK, Arora VK. Artificial intelligence and TB management - The way forward. Indian J Tuberc. 2020;67:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (2)] |
| 21. | Doshi R, Falzon D, Thomas BV, Temesgen Z, Sadasivan L, Migliori GB, Raviglione M. Tuberculosis control, and the where and why of artificial intelligence. ERJ Open Res. 2017;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 22. | Li L, Qin L, Xu Z, Yin Y, Wang X, Kong B, Bai J, Lu Y, Fang Z, Song Q, Cao K, Liu D, Wang G, Xu Q, Fang X, Zhang S, Xia J. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology. 2020;296:E65-E71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1260] [Cited by in RCA: 946] [Article Influence: 157.7] [Reference Citation Analysis (13)] |
| 23. | Zhu J, Shen B, Abbasi A, Hoshmand-Kochi M, Li H, Duong TQ. Deep transfer learning artificial intelligence accurately stages COVID-19 Lung disease severity on portable chest radiographs. PLoS One. 2020;15:e0236621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (2)] |
| 24. | Burdick H, Lam C, Mataraso S, Siefkas A, Braden G, Dellinger RP, McCoy A, Vincent JL, Green-Saxena A, Barnes G, Hoffman J, Calvert J, Pellegrini E, Das R. Prediction of respiratory decompensation in Covid-19 patients using machine learning: The READY trial. Comput Biol Med. 2020;124:103949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 25. | Binczyk F, Prazuch W, Bozek P, Polanska J. Radiomics and artificial intelligence in lung cancer screening. Transl Lung Cancer Res. 2021;10:1186-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 26. | Zhang Y, Osanlouy M, Clark AR, Kumar H, King C, Wilsher ML, Milne DG, Hoffman EA, Tawhai MH. Pulmonary lobar segmentation from computed tomography scans based on a statistical finite element analysis of lobe shape. Proc. SPIE 10949, Medical Imaging 2019. [DOI] [Full Text] |
| 27. | Chauvie S, De Maggi A, Baralis I, Dalmasso F, Berchialla P, Priotto R, Violino P, Mazza F, Melloni G, Grosso M; SOS Study team. Artificial intelligence and radiomics enhance the positive predictive value of digital chest tomosynthesis for lung cancer detection within SOS clinical trial. Eur Radiol. 2020;30:4134-4140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 28. | Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 4201] [Article Influence: 300.1] [Reference Citation Analysis (4)] |
| 29. | Afshar P, Mohammadi A, Tyrrell PN, Cheung P, Sigiuk A, Plataniotis KN, Nguyen ET, Oikonomou A. [Formula: see text]: deep learning-based radiomics for the time-to-event outcome prediction in lung cancer. Sci Rep. 2020;10:12366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 30. | Schreuder A, Scholten ET, van Ginneken B, Jacobs C. Artificial intelligence for detection and characterization of pulmonary nodules in lung cancer CT screening: ready for practice? Transl Lung Cancer Res. 2021;10:2378-2388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 31. | Belfiore MP, Urraro F, Grassi R, Giacobbe G, Patelli G, Cappabianca S, Reginelli A. Artificial intelligence to codify lung CT in Covid-19 patients. Radiol Med. 2020;125:500-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Hanada E, Morilla I, Muneer A, Wan XH, Wang P, Zhang W S-Editor: Liu JH L-Editor: A P-Editor: Liu JH