Published online Aug 28, 2020. doi: 10.35711/aimi.v1.i2.78
Peer-review started: June 5, 2020
First decision: June 4, 2020
Revised: August 1, 2020
Accepted: August 22, 2020
Article in press: August 22, 2020
Published online: August 28, 2020
Processing time: 94 Days and 0.9 Hours
Optical molecular tomography (OMT) is an imaging modality which uses an optical signal, especially near-infrared light, to reconstruct the three-dimensional information of the light source in biological tissue. With the advantages of being low-cost, noninvasive and having high sensitivity, OMT has been applied in preclinical and clinical research. However, due to its serious ill-posedness and ill-condition, the solution of OMT requires heavy data analysis and the reconstruction quality is limited. Recently, the artificial intelligence (commonly known as AI)-based methods have been proposed to provide a different tool to solve the OMT problem. In this paper, we review the progress on OMT algorithms, from conventional methods to AI-based methods, and we also give a prospective towards future developments in this domain.
Core Tip: Most of the existing review articles about optical molecular tomography (OMT) focus on the traditional light propagation model-based algorithm, which possesses ill-posedness and ill-condition and the reconstruction result is unsatisfactory. The emergence of deep learning has brought OMT into the era of artificial intelligence, which can obtain a highly accurate reconstruction result. This article systematically reviews the development of tomographic reconstruction for OMT, which involves the light propagation model-based OMT algorithm and machine learning-based OMT algorithm. The challenges and perspectives of these machine learning-based algorithms are given at the end of the article.
- Citation: Cao X, Li K, Xu XL, Deneen KMV, Geng GH, Chen XL. Development of tomographic reconstruction for three-dimensional optical imaging: From the inversion of light propagation to artificial intelligence. Artif Intell Med Imaging 2020; 1(2): 78-86
- URL: https://www.wjgnet.com/2644-3260/full/v1/i2/78.htm
- DOI: https://dx.doi.org/10.35711/aimi.v1.i2.78
Optical molecular imaging (OMI) is the technology of using optical imaging instruments to detect biological tissues in organisms. In the time since Roger Yonchien Tsien reported that the tumor of a mouse could be resected under the guidance of fluorescence microscopy, winning the Nobel Prize in 2008, OMI has achieved rapid development, especially in recent years. With the advantages of high imaging sensitivity, tissue specificity, relatively short acquisition time and low cost, OMI has been successfully applied to many research fields, including - but not limited to - gene expression, tumor detection, drug development, and therapy evaluation[1-12]. However, OMI can only provide a two-dimensional image, which lacks deeper information and cannot describe the 3D distribution of the optical signal in an imaging object. Thus, researchers have proposed a series of 3D imaging methods, which can be named as optical molecular tomography (OMT).
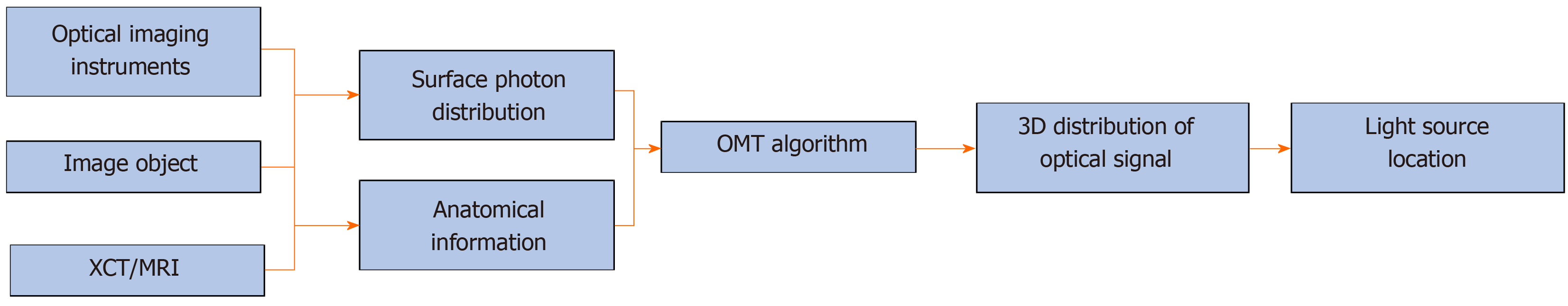
In fact, OMT can be further divided into several subtypes, such as bioluminescence tomography, Cerenkov luminescence tomography (CLT), fluorescence molecular tomography (FMT), diffuse optical tomography, X-ray luminescence computed tomography (commonly referred to as XLCT), and so on[13-18]. The main difference between them is the means of producing the optical signal. For example, in CLT, the optical signal is emitted during the decay of a radionuclide probe, and in XLCT, high energy X-ray photons are used to excite X-ray excitable nanophosphors which emit the optical signal. Although the way of producing light signal varies, the reconstruction methods for these modalities can be concluded as one unified framework, as shown in Figure 1. It should be noted that anatomical information is essential for OMT, and in most cases, it is provided by X-ray computed tomography or magnetic resonance imaging[19-21]. Finally, the 3D distribution of the optical signal in the imaging object can be obtained, and the light source can then be located based on the reconstruction result. It is obvious that the core component of the framework is the OMT algorithm, which can determine the quality of the final reconstruction result.
In this review, we summarize recent progress on the OMT algorithm in two aspects: the traditional light propagation model-based way and machine learning-based way. Subsequently, we will provide a prospect towards future developments in a machine learning-based way for OMT.
The accuracy of the traditional OMT algorithm is dependent on the description of photon propagation in biological tissue. The most popular light transfer model for OMT is the radiative transfer equation (RTE) from Maxwell’s equations[22-25]. Although RTE can accurately depict photon propagation in diffusive media, it is a complicated integro-differential equation, and the computational time and memory requirements are extremely expensive. As a result, RTE is commonly simplified as the diffusion equation (DE, the lower-order approximation of the RTE)[26-28] and Nrd order is a simplified spherical harmonics function (SP3, the high-order approximation of RTE, and in most cases, N equals 3)[29-31]. After introducing the boundary condition, the simplified RTE can be solved using the finite element method[32-34] and the OMT problem can be linearized as the following weight matrix equation[28,25-37]: AX = Φmeasure, where X ≥ 0 (Eq. 1) "where A" denotes the optical transport system matrix, X is the unknown distribution of the optical source and Φmeasure represents the luminous flux of the vertices. As Φmeasure can only be collected on the surface of an imaging object, the goal of OMT can be regarded as the determination of the 3D luminescence source distribution X from boundary measurements Φmeasure based on the formulation of Eq. 1, and this is a typically inverse problem. It should be noted that the number of measurements is often substantially less than the number of unknowns, making the inverse reconstruction an ill-conditioned problem.
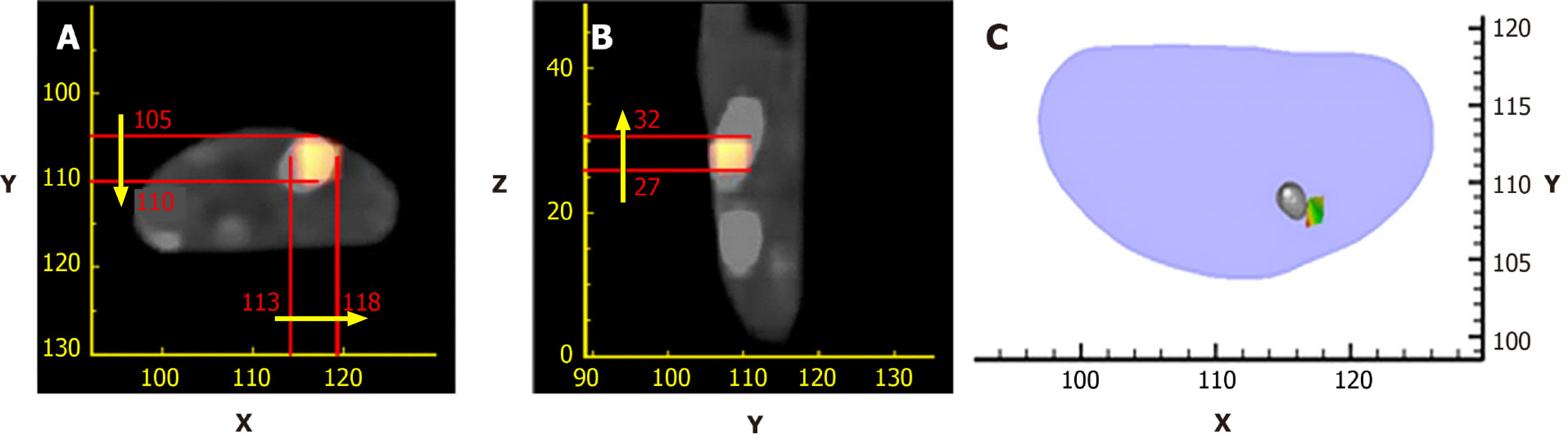
Up until now, many methods have been developed to address the limitation mentioned above to make the OMT algorithm strong and robust. These methods can be roughly divided into two categories. The first one is the priori information-based method. In these methods, a priori information is first inferred according to the surface light power distribution and the heterogeneous structure of the imaging object, and then is used as the permissible source region. The aim of using a priori information is to constrain the unknown sources in the region where the sources may exist, resulting in the reduction of the amount of unknown source locations. Many numerical and in vivo experiments have been conducted, and the results indicate that the size of the permissible source region can significantly affect the reconstruction quality[37-46]. It is obvious that the smaller the permissible source region, the more stable the reconstruction results. The main obstacle of the priori information-based methods is that the prior information about the permissible source region cannot always be obtained in advance, especially for the early diseased tissue which cannot be distinguished from anatomical information. Figure 2 shows the reconstruction results with a priori information[47].
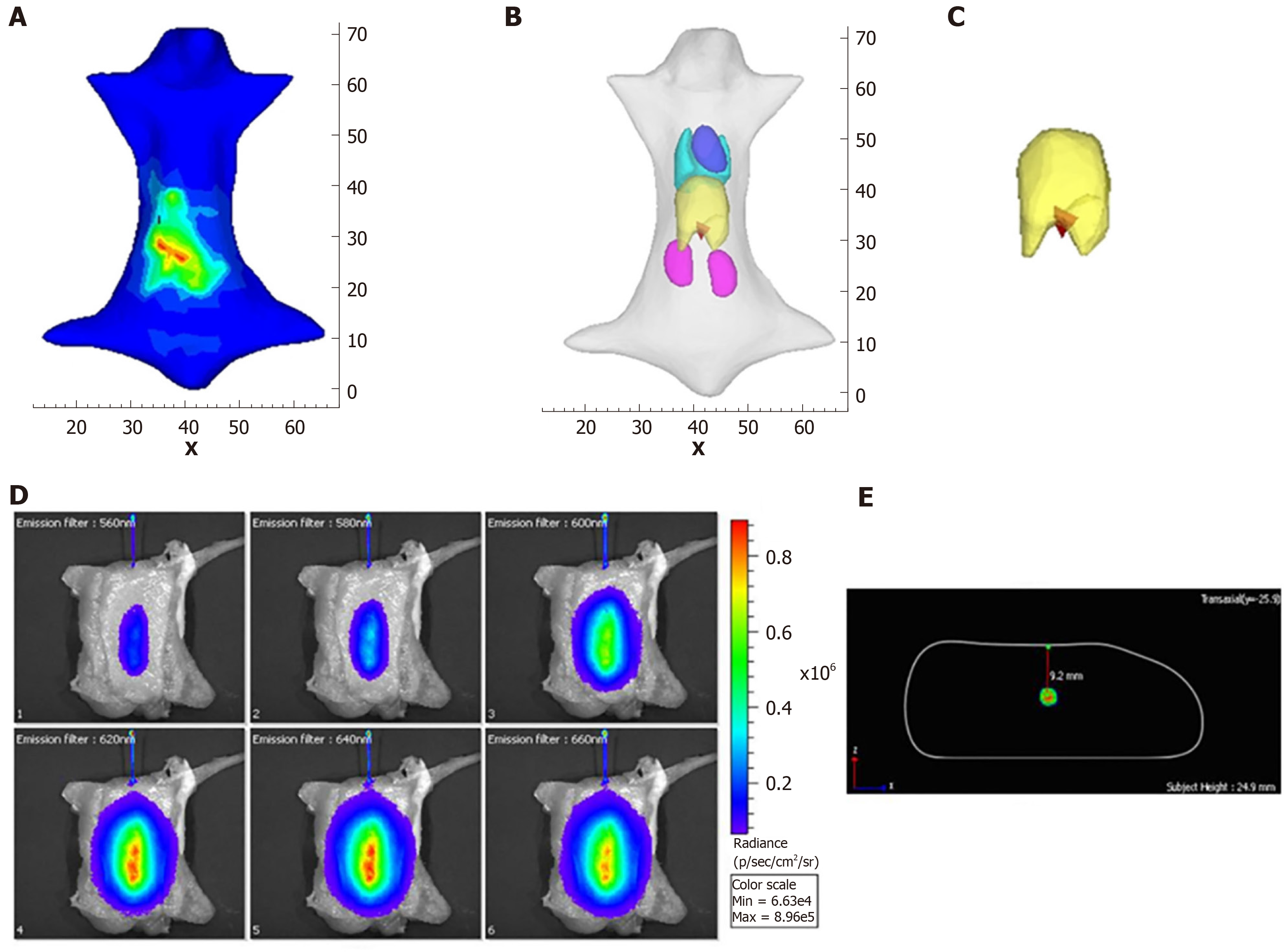
The second one is the posteriori information-based method. In these methods, the whole object is used as the initial permissible source region, and the permissible region is updated by selecting the elements where the reconstructed energy is relatively higher than others[48-53]. As the posteriori information-based method avoids the segmentation of the permissible source region from anatomical information, it has superior generalization performance than the priori information-based method, and most of the recent studies are focused on optimizing it[54-58]. Besides the above methods for OMT, the reconstruction accuracy can also be improved by increasing the number of detectable measurements[46,59-66]. For example, in FMT, the quality of the reconstructed results can be improved with the increasing number of measurement data. In CLT, multispectral images can be acquired using a group of filters and the result can be improved significantly. The drawback of this method is that the more optical signal data are acquired, the more time is consumed. However, these traditional light propagation model-based methods are still limited to their reconstruction accuracy, and the main reason is that the simplified RTE cannot accurately describe the process of photon propagation. Thus, more effective methods to improve the reconstruction quality of OMT are still required. Figure 3 shows the reconstruction results with posteriori information[62,67].
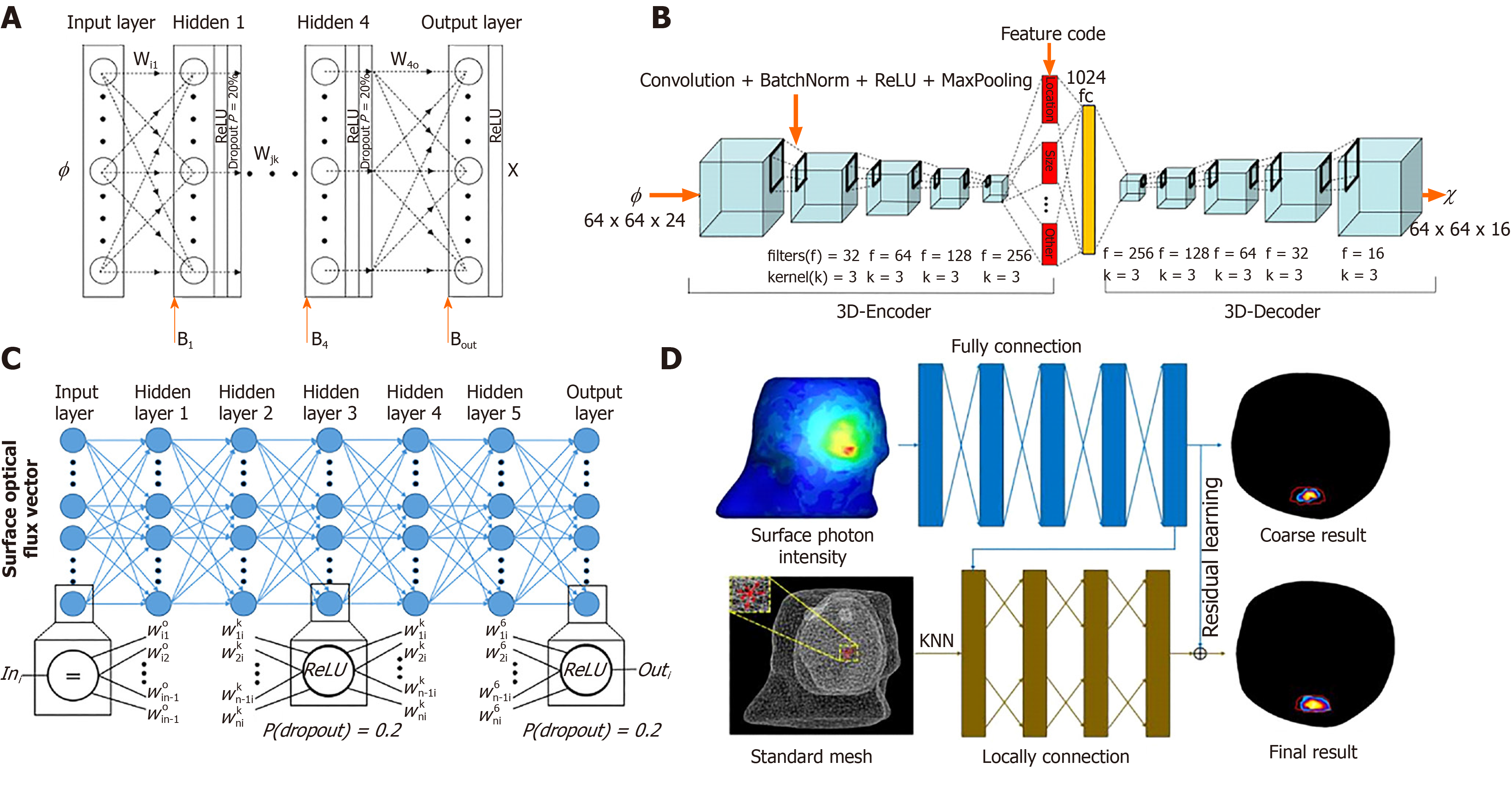
With the development of artificial intelligence (AI), machine learning algorithms, especially deep learning-based technologies, have gained stunning successes at solving difficult and previously unsolved computational problems in many fields, such as computer vision, natural language processing, speech recognition, and so on[68-71]. The great success of AI has also attracted the attention of researchers in the field of OMT. Based on multilayer perceptrons (commonly known as MLPs), Gao et al[72] proposed a data-driven-based strategy for OMT. As the machine learning-based method requires large amounts of data to train the network, Molecular Optical Simulation Environment software[73] is adopted to produce the simulation data. The experimental results showed the proposed method can greatly improve the reconstruction quality compared with conventional approaches. Subsequently, based on the convolutional neural network and recurrent neural network, Guo et al[74] proposed a framework for FMT reconstruction. The input of this method is two-dimensional fluorescent images, which can avoid errors caused by mesh registration in conventional methods. Zhang et al[75] used MLPs to solve the CLT problem, and the complex relationship between the surface optical signal and the true photon source has been learned by the network. Meng et al[76] constructed a K-nearest neighbor-based locally connected network (KNN-LCN) for FMT. In their work, KNN-LCN cascades a fully connected (referred to as FC) sub-network with a locally connected (referred to as LC) sub-network, where the FC part provides a coarse reconstruction result and the LC part fine-tunes the morphological quality of the reconstructed result. Compared to the traditional light propagation model-based methods, the biggest advantage of the machine learning-based method is that it can directly fit the nonlinear relationship between an object surface optical density and its internal luminescence source. Figure 4 shows the structure of the networks used in OMT reconstruction[72,74-76].
Although the machine learning-based OMT algorithm can obtain a more accurate reconstruction result than the traditional light propagation model-based algorithm, further application is still limited and requires more theoretical research. One reason is that the network trained for one object cannot be used for others, and if the object is changed, another network with different parameters should be built and its training will cost a lot of time. Another reason is that there is no ideal method that can explain the mechanism of such a neural network. The solution to the above two limitations is the development direction for future research. In addition, there are many environmental, dietary, and other factors that influence the microbiome, immune system, and pathogenic mechanisms. The recent studies on molecular pathological epidemiology have provided a powerful tool which can pathologically, epidemiologically investigate those factors in relation to molecular pathologies, immunity, and clinical outcomes[77], and it is believed that the molecular pathological epidemiology research can be a promising direction and in which OMT can take a big role.
| 1. | Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1963] [Cited by in RCA: 1674] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 2. | Nguyen QT, Tsien RY. Fluorescence-guided surgery with live molecular navigation--a new cutting edge. Nat Rev Cancer. 2013;13:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 441] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 3. | Hu Z, Qu Y, Wang K, Zhang X, Zha J, Song T, Bao C, Liu H, Wang Z, Wang J, Liu Z, Liu H, Tian J. In vivo nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging. Nat Commun. 2015;6:7560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Klose AD, Paragas N. Automated quantification of bioluminescence images. Nat Commun. 2018;9:4262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Thorek DL, Ogirala A, Beattie BJ, Grimm J. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat Med. 2013;19:1345-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Zhang Z, Cai M, Bao C, Hu Z, Tian J. Endoscopic Cerenkov luminescence imaging and image-guided tumor resection on hepatocellular carcinoma-bearing mouse models. Nanomedicine. 2019;17:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Mitchell GS, Lloyd PNT, Cherry SR. Cerenkov luminescence and PET imaging of 90Y: capabilities and limitations in small animal applications. Phys Med Biol. 2020;65:065006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Cao X, Zhan Y, Cao X, Liang J, Chen X. Harnessing the Power of Cerenkov Luminescence Imaging for Gastroenterology: Cerenkov Luminescence Endoscopy. Curr Med Imaging Rev. 2017;13:50-57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Cao X, Chen X, Kang F, Zhan Y, Cao X, Wang J, Liang J, Tian J. Intensity Enhanced Cerenkov Luminescence Imaging Using Terbium-Doped Gd2O2S Microparticles. ACS Appl Mater Interfaces. 2015;7:11775-11782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Cao X, Chen X, Kang F, Cao X, Zhan Y, Wang J, Wu K, Liang J. Sensitivity improvement of Cerenkov luminescence endoscope with terbium doped Gd2O2S nanoparticles. Appl Phys Lett. 2015;106:4. [DOI] [Full Text] |
| 11. | Cao X, Chen X, Kang F, Lin Y, Liu M, Hu H, Nie Y, Wu K, Wang J, Liang J, Tian J. Performance evaluation of endoscopic Cerenkov luminescence imaging system: in vitro and pseudotumor studies. Biomed Opt Express. 2014;5:3660-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Fan D, Zhang X, Zhong L, Liu X, Sun Y, Zhao H, Jia B, Liu Z, Zhu Z, Shi J, Wang F. (68)Ga-labeled 3PRGD2 for dual PET and Cerenkov luminescence imaging of orthotopic human glioblastoma. Bioconjug Chem. 2015;26:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Gao Y, Wang K, Jiang S, Liu Y, Ai T, Tian J. Corrections to "Bioluminescence Tomography Based on Gaussian Weighted Laplace Prior Regularization for Morphological Imaging of Glioma". IEEE Trans Med Imaging. 2018;37:1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Zhong J, Qin C, Yang X, Zhu S, Zhang X, Tian J. Cerenkov luminescence tomography for in vivo radiopharmaceutical imaging. Int J Biomed Imaging. 2011;2011:641618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Zhang B, Liu S, Cao X, Liu F, Wang X, Luo J, Shan B, Bai J. Fluorescence Tomography Reconstruction With Simultaneous Positron Emission Tomography Priors. IEEE Trans Multimedia. 2013;15:1031-1038. [DOI] [Full Text] |
| 16. | Baritaux JC, Hassler K, Bucher M, Sanyal S, Unser M. Sparsity-driven reconstruction for FDOT with anatomical priors. IEEE Trans Med Imaging. 2011;30:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Lewis MA, Kodibagkar VD, Öz OK, Mason RP. On the potential for molecular imaging with Cerenkov luminescence. Opt Lett. 2010;35:3889-3891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002;4:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 634] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 19. | Meng H, Wang K, Gao Y, Jin Y, Ma X, Tian J. Adaptive Gaussian Weighted Laplace Prior Regularization Enables Accurate Morphological Reconstruction in Fluorescence Molecular Tomography. IEEE Trans Med Imaging. 2019;38:2726-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Guo H, Hou Y, He X, Yu J, Cheng J, Pu X. Adaptive hp finite element method for fluorescence molecular tomography with simplified spherical harmonics approximation. J Innov Opt Health Sci. 2014;7:12. [DOI] [Full Text] |
| 21. | Yi H, Wei H, Peng J, Hou Y, He X. Adaptive threshold method for recovered images of FMT. J Opt Soc Am A Opt Image Sci Vis. 2018;35:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Cai W, Xu M, Alfano R. Three-dimensional radiative transfer tomography for turbid media. IEEE J Sel Top Quantum Electron. 2003;9:189-198. [DOI] [Full Text] |
| 23. | Joshi A, Rasmussen JC, Sevick-Muraca EM, Wareing TA, McGhee J. Radiative transport-based frequency-domain fluorescence tomography. Phys Med Biol. 2008;53:2069-2088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Gao H, Zhao H. Multilevel bioluminescence tomography based on radiative transfer equation Part 1: l1 regularization. Opt Express. 2010;18:1854-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Klose AD. The forward and inverse problem in tissue optics based on the radiative transfer equation: a brief review. J Quant Spectrosc Radiat Transf. 2010;111:1852-1853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Wang G, Cong W, Durairaj K, Qian X, Shen H, Sinn P, Hoffman E, McLennan G, Henry M. In vivo mouse studies with bioluminescence tomography. Opt Express. 2006;14:7801-7809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Cong W, Wang G, Kumar D, Liu Y, Jiang M, Wang L, Hoffman E, McLennan G, McCray P, Zabner J, Cong A. Practical reconstruction method for bioluminescence tomography. Opt Express. 2005;13:6756-6771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Li C, Mitchell GS, Cherry SR. Cerenkov luminescence tomography for small-animal imaging. Opt Lett. 2010;35:1109-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Yang D, Chen X, Cao X, Wang J, Liang J, Tian J. Performance investigation of SP3 and diffusion approximation for three-dimensional whole-body optical imaging of small animals. Med Biol Eng Comput. 2015;53:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Zhong J, Tian J, Yang X, Qin C. Whole-body Cerenkov luminescence tomography with the finite element SP(3) method. Ann Biomed Eng. 2011;39:1728-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Zhong J, Tian J, Yang X, Qin C. L1-regularized Cerenkov luminescence tomography with a SP3 method and CT fusion. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6158-6161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Han D, Tian J, Zhu S, Feng J, Qin C, Zhang B, Yang X. A fast reconstruction algorithm for fluorescence molecular tomography with sparsity regularization. Opt Express. 2010;18:8630-8646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Chen X, Gao X, Chen D, Ma X, Zhao X, Shen M, Li X, Qu X, Liang J, Ripoll J, Tian J. 3D reconstruction of light flux distribution on arbitrary surfaces from 2D multi-photographic images. Opt Express. 2010;18:19876-19893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Han R, Liang J, Qu X, Hou Y, Ren N, Mao J, Tian J. A source reconstruction algorithm based on adaptive hp-FEM for bioluminescence tomography. Opt Express. 2009;17:14481-14494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Lu Y, Zhu B, Shen H, Rasmussen JC, Wang G, Sevick-Muraca EM. A parallel adaptive finite element simplified spherical harmonics approximation solver for frequency domain fluorescence molecular imaging. Phys Med Biol. 2010;55:4625-4645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Liu K, Lu Y, Tian J, Qin C, Yang X, Zhu S, Yang X, Gao Q, Han D. Evaluation of the simplified spherical harmonics approximation in bioluminescence tomography through heterogeneous mouse models. Opt Express. 2010;18:20988-21002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Liu J, Wang Y, Qu X, Li X, Ma X, Han R, Hu Z, Chen X, Sun D, Zhang R, Chen D, Chen D, Chen X, Liang J, Cao F, Tian J. In vivo quantitative bioluminescence tomography using heterogeneous and homogeneous mouse models. Opt Express. 2010;18:13102-13113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Baikejiang R, Zhao Y, Fite BZ, Ferrara KW, Li C. Anatomical image-guided fluorescence molecular tomography reconstruction using kernel method. J Biomed Opt. 2017;22:55001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Holt RW, Demers JL, Sexton KJ, Gunn JR, Davis SC, Samkoe KS, Pogue BW. Tomography of epidermal growth factor receptor binding to fluorescent Affibody in vivo studied with magnetic resonance guided fluorescence recovery in varying orthotopic glioma sizes. J Biomed Opt. 2015;20:26001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Davis SC, Samkoe KS, Tichauer KM, Sexton KJ, Gunn JR, Deharvengt SJ, Hasan T, Pogue BW. Dynamic dual-tracer MRI-guided fluorescence tomography to quantify receptor density in vivo. Proc Natl Acad Sci U S A. 2013;110:9025-9030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Qin C, Zhu S, Feng J, Zhong J, Ma X, Wu P, Tian J. Comparison of permissible source region and multispectral data using efficient bioluminescence tomography method. J Biophotonics. 2011;4:824-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Ma X, Tian J, Qin C, Yang X, Zhang B, Xue Z, Zhang X, Han D, Dong D, Liu X. Early detection of liver cancer based on bioluminescence tomography. Appl Opt. 2011;50:1389-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Huang H, Qu X, Liang J, He X, Chen X, Yang D, Tian J. A multi-phase level set framework for source reconstruction in bioluminescence tomography. J Comput Phys. 2010;229:5246-5256. [DOI] [Full Text] |
| 44. | Davis SC, Samkoe KS, O'Hara JA, Gibbs-Strauss SL, Paulsen KD, Pogue BW. Comparing implementations of magnetic-resonance-guided fluorescence molecular tomography for diagnostic classification of brain tumors. J Biomed Opt. 2010;15:051602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Schulz RB, Ale A, Sarantopoulos A, Freyer M, Soehngen E, Zientkowska M, Ntziachristos V. Hybrid system for simultaneous fluorescence and x-ray computed tomography. IEEE Trans Med Imaging. 2010;29:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Feng J, Jia K, Yan G, Zhu S, Qin C, Lv Y, Tian J. An optimal permissible source region strategy for multispectral bioluminescence tomography. Opt Express. 2008;16:15640-15654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Hu Z, Chen X, Liang J, Qu X, Chen D, Yang W, Wang J, Cao F, Tian J. Single photon emission computed tomography-guided Cerenkov luminescence tomography. J Appl Phys. 2012;112:024703. [DOI] [Full Text] |
| 48. | Chehade M, Srivastava AK, Bulte JW. Co-Registration of Bioluminescence Tomography, Computed Tomography, and Magnetic Resonance Imaging for Multimodal In Vivo Stem Cell Tracking. Tomography. 2016;2:159-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Zhang X, Lu Y, Chan T. A novel sparsity reconstruction method from Poisson data for 3D bioluminescence tomography. J Sci Comput. 2012;50:519-535. [DOI] [Full Text] |
| 50. | Dutta J, Ahn S, Li C, Cherry SR, Leahy RM. Joint L1 and total variation regularization for fluorescence molecular tomography. Phys Med Biol. 2012;57:1459-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Liu K, Tian J, Qin C, Yang X, Zhu S, Han D, Wu P. Tomographic bioluminescence imaging reconstruction via a dynamically sparse regularized global method in mouse models. J Biomed Opt. 2011;16:046016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Lu Y, Zhang X, Douraghy A, Stout D, Tian J, Chan TF, Chatziioannou AF. Source reconstruction for spectrally-resolved bioluminescence tomography with sparse a priori information. Opt Express. 2009;17:8062-8080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Xu X, Deng Z, Iordachita I, Wong J, Wang K. A Novel Multi-Projection Bioluminescence Tomography for Small Animal Radiation Research Platform (SARRP). Med Phys. 2018;45:E393-E393. |
| 54. | Guo H, Gao L, Yu J, He X, Wang H, Zheng J, Yang X. Sparse-graph manifold learning method for bioluminescence tomography. J Biophotonics. 2020;13:e201960218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Cai M, Zhang Z, Shi X, Yang J, Hu Z, Tian J. Non-negative Iterative Convex Refinement Approach for Accurate and Robust Reconstruction in Cerenkov Luminescence Tomography. IEEE Trans Med Imaging. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Wang L, Cao H, Cao X, Ren S, Li K, Zhan Y, Chen X, He X. Adaptively Hybrid 3 Simplified Spherical Harmonics With Diffusion Equation-Based Multispectral Cerenkov Luminescence Tomography. IEEE Access. 2019;7:160779-160785. [DOI] [Full Text] |
| 57. | Pu H, Gao P, Liu Y, Rong J, Shi F, Lu H. Principal Component Analysis Based Dynamic Cone Beam X-Ray Luminescence Computed Tomography: A Feasibility Study. IEEE Trans Med Imaging. 2019;38:2891-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Liu X, Tang X, Shu Y, Zhao L, Liu Y, Zhou T. Single-view cone-beam x-ray luminescence optical tomography based on Group_YALL1 method. Phys Med Biol. 2019;64:105004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | He X, Xiang W, Yu J, Li Q. Penalty method for source reconstruction of multispectral bioluminescence tomography. Opt Eng. 2018;57:083104. [DOI] [Full Text] |
| 60. | Guo H, He X, Liu M, Zhang Z, Hu Z, Tian J. Weight Multispectral Reconstruction Strategy for Enhanced Reconstruction Accuracy and Stability With Cerenkov Luminescence Tomography. IEEE Trans Med Imaging. 2017;36:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Liu H, Yang X, Song T, Bao C, Shi L, Hu Z, Wang K, Tian J. Multispectral hybrid Cerenkov luminescence tomography based on the finite element SPn method. J Biomed Opt. 2015;20:86007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Spinelli AE, Kuo C, Rice BW, Calandrino R, Marzola P, Sbarbati A, Boschi F. Multispectral Cerenkov luminescence tomography for small animal optical imaging. Opt Express. 2011;19:12605-12618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 63. | Li C, Yang Y, Mitchell GS, Cherry SR. Simultaneous PET and multispectral 3-dimensional fluorescence optical tomography imaging system. J Nucl Med. 2011;52:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Crane LM, Themelis G, Pleijhuis RG, Harlaar NJ, Sarantopoulos A, Arts HJ, van der Zee AG, Ntziachristos V, van Dam GM. Intraoperative multispectral fluorescence imaging for the detection of the sentinel lymph node in cervical cancer: a novel concept. Mol Imaging Biol. 2011;13:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 65. | Chaudhari AJ, Darvas F, Bading JR, Moats RA, Conti PS, Smith DJ, Cherry SR, Leahy RM. Hyperspectral and multispectral bioluminescence optical tomography for small animal imaging. Phys Med Biol. 2005;50:5421-5441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 66. | Tong S, Han B, Chen Y, Tang J, Bi B, Gu R. RTE-based parameter reconstruction with TV+L1 regularization. J Comput Appl Math. 2018;337:256-273. [DOI] [Full Text] |
| 67. | Yang D, Wang L, Chen D, Yan C, He X, Liang J, Chen X. Filtered maximum likelihood expectation maximization based global reconstruction for bioluminescence tomography. Med Biol Eng Comput. 2018;56:2067-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Gurovich Y, Hanani Y, Bar O, Nadav G, Fleischer N, Gelbman D, Basel-Salmon L, Krawitz PM, Kamphausen SB, Zenker M, Bird LM, Gripp KW. Identifying facial phenotypes of genetic disorders using deep learning. Nat Med. 2019;25:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 421] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 69. | Hannun AY, Rajpurkar P, Haghpanahi M, Tison GH, Bourn C, Turakhia MP, Ng AY. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1018] [Cited by in RCA: 1270] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 70. | Moen E, Bannon D, Kudo T, Graf W, Covert M, Van Valen D. Deep learning for cellular image analysis. Nat Methods. 2019;16:1233-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 665] [Article Influence: 95.0] [Reference Citation Analysis (10)] |
| 71. | LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36149] [Cited by in RCA: 21192] [Article Influence: 1926.5] [Reference Citation Analysis (2)] |
| 72. | Gao Y, Wang K, An Y, Jiang S, Meng H, Tian J. Nonmodel-based bioluminescence tomography using a machine-learning reconstruction strategy. Optica. 2018;5:1451-1454. [DOI] [Full Text] |
| 73. | Ren S, Chen X, Wang H, Qu X, Wang G, Liang J, Tian J. Molecular Optical Simulation Environment (MOSE): a platform for the simulation of light propagation in turbid media. PLoS One. 2013;8:e61304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Guo L, Liu F, Cai C, Liu J, Zhang G. 3D deep encoder-decoder network for fluorescence molecular tomography. Opt Lett. 2019;44:1892-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 75. | Zhang Z, Cai M, Gao Y, Shi X, Zhang X, Hu Z, Tian J. A novel Cerenkov luminescence tomography approach using multilayer fully connected neural network. Phys Med Biol. 2019;64:245010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 76. | Meng H, Gao Y, Yang X, Wang K, Tian J. K-nearest Neighbor Based Locally Connected Network for Fast Morphological Reconstruction in Fluorescence Molecular Tomography. IEEE Trans Med Imaging. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Ogino S, Lochhead P, Chan AT, Nishihara R, Cho E, Wolpin BM, Meyerhardt JA, Meissner A, Schernhammer ES, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol. 2013;26:465-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Engineering, biomedical
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nassar G, Ogino S S-Editor: Wang JL L-Editor: Filipodia P-Editor: Xing YX