Published online Jan 8, 2026. doi: 10.35712/aig.v7.i1.115054
Revised: October 28, 2025
Accepted: December 1, 2025
Published online: January 8, 2026
Processing time: 92 Days and 1.6 Hours
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal neoplasms pri
A 40-year-old male with jaundice and abdominal symptoms underwent imaging, which suggested a malignant periampullary tumor. Preoperative misdiagnosis of pancreatic cancer was made, and surgery was performed. Postoperative histo
Artificial intelligence in radiomic imaging holds significant promise in enhancing the diagnostic process for rare cancers like duodenal GISTs, ensuring timely and accurate treatment.
Core Tip: Duodenal gastrointestinal stromal tumors (GISTs) are rare and often mimic periampullary or pancreatic tumors, leading to misdiagnosis, especially when presenting with obstructive jaundice. This case highlights how artificial intelligence (AI) could significantly enhance preoperative diagnosis by analyzing subtle imaging features that differentiate GISTs from other malignancies. AI-driven radiomics and deep learning models can improve tumor characterization, predict biological behavior, and guide timely treatment decisions. Integrating AI into diagnostic workflows may prevent unnecessary major surgeries and improve outcomes in rare gastrointestinal tumors like duodenal GISTs.
- Citation: Agrawal H, Dwivedi G, Rohitaj R, Tanwar H, Maurya S, Gupta N. Integrating artificial intelligence in the diagnostic pathway of duodenal gastrointestinal stromal tumors: A case report. Artif Intell Gastroenterol 2026; 7(1): 115054
- URL: https://www.wjgnet.com/2644-3236/full/v7/i1/115054.htm
- DOI: https://dx.doi.org/10.35712/aig.v7.i1.115054
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal neoplasms originating from the interstitial cells of Cajal, most commonly found in the stomach and small intestine. However, duodenal GISTs are particularly rare, making up a small proportion of all GIST cases[1]. The symptoms of GISTs are often nonspecific, with common presentations including abdominal pain, gastrointestinal bleeding, and obstruction[2]. In some instances, GISTs may present with atypical symptoms, such as obstructive jaundice, which can easily lead to a misdiagnosis, as seen in the case discussed here.
Recent advances in artificial intelligence (AI) offer significant promise in the diagnostic process for complex conditions like GISTs. AI-powered tools, including machine learning algorithms and radiomic analysis, can enhance the accuracy of imaging, improving the early detection and characterization of tumors[3]. In particular, AI can assist in preoperative diagnosis by analyzing imaging data such as computed tomography scans and endoscopic images to detect subtle tumor characteristics that human radiologists may overlook. AI’s predictive capabilities could provide better insights into the tumor’s nature, thereby guiding clinicians towards more informed, timely interventions. In the case of duodenal GISTs, AI could have played a pivotal role in identifying the tumor preoperatively, potentially predicting the tumor’s behavior and risk, which would have influenced treatment decisions[4].
This case illustrates the critical role AI could have played in the early diagnosis of a duodenal GIST, which was only confirmed postoperatively via biopsy. The case highlights how AI could have potentially improved the preoperative diagnostic process and predicted a better clinical outcome for the patient.
A 40-year-old male patient presented with a one-month history of anorexia, intermittent jaundice, itching, clay-colored stools, and fever episodes.
A 40-year-old male patient presented with a one-month history of anorexia, intermittent jaundice, and itching. The jaundice has been fluctuating in intensity, with a yellowish discoloration of the skin and sclera. The itching has been generalized, predominantly affecting the palms and soles, and is most noticeable during the evenings. He reports passing clay-colored stools and has had episodes of fever, which are sporadic and associated with chills but without significant sweating. There has been no recent history of nausea, vomiting, or abdominal pain. He denies weight loss, changes in urine color, or any history of recent travel, alcohol use, or known liver disease.
No significant past medical history.
Negative for any significant personal or family medical history.
On physical examination, the patient had jaundice (icterus), a palpable gallbladder, and a firm 4 cm× 4 cm mass in the epigastrium. His vital signs were stable, with a pulse rate of 60 beats per minute, blood pressure of 114/70 mmHg, and oxygen saturation of 98% on room air.
Laboratory investigations revealed significant anemia (hemoglobin of 6.4 g/dL), elevated white blood cell count (19000/μL), and liver dysfunction (total bilirubin of 5.4 mg/dL and direct bilirubin of 2.7 mg/dL).
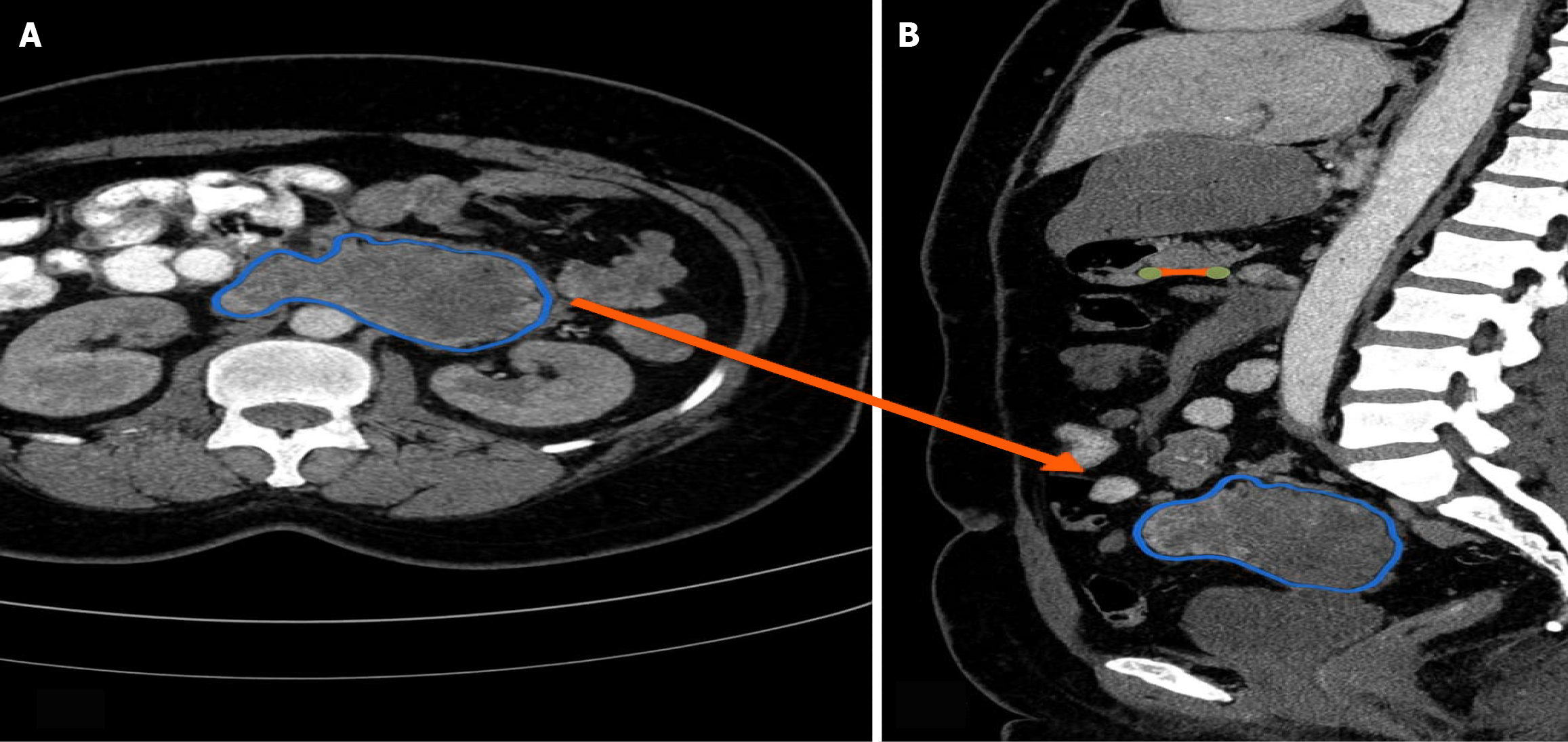
Imaging studies, including a triple-phase contrast-enhanced computed tomography scan, revealed a large lesion near the duodenal C-loop, which appeared to originate from the pancreas head and encased the common bile duct, pancreatic duct, and duodenum (Figure 1). The lesion caused intrahepatic bile duct dilatation and extended into the gallbladder fossa, suggesting a malignant periampullary tumor, likely pancreatic cancer.
Given the preoperative imaging results, the clinical suspicion was pancreatic cancer.
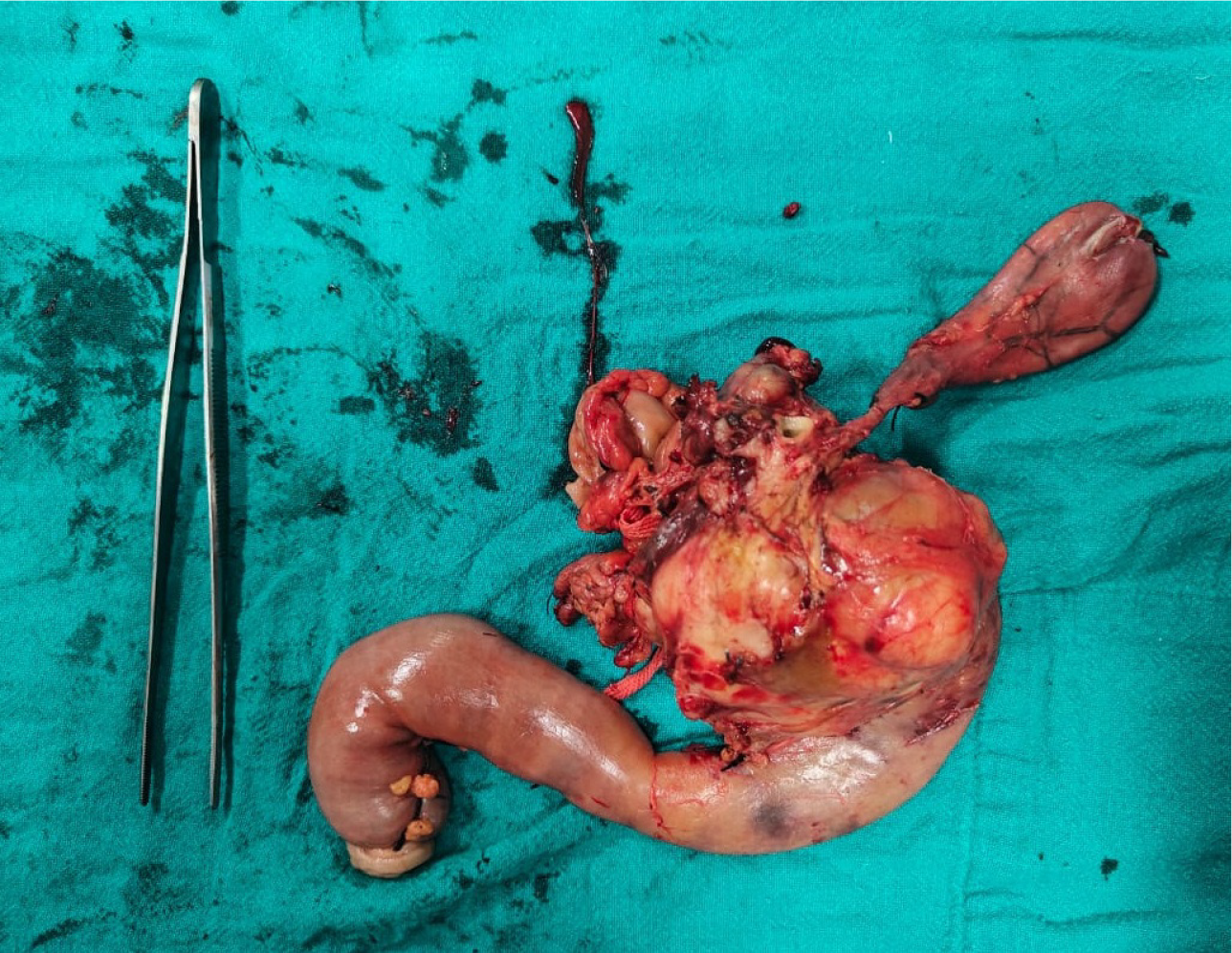
The patient underwent a Whipple’s procedure (pancreaticoduodenectomy) with feeding jejunostomy (Figure 2).
The surgery was well-tolerated, and postoperative recovery was uneventful, with drains removed on post-operative days 7 and days 10. However, the definitive diagnosis was only established postoperatively upon histopathological examination, which revealed a duodenal GIST, measuring 10.5 cm × 9 cm × 8 cm, and demonstrating high mitotic activity. The tumor was free from the duodenal margins, uncinate process, and pancreatic neck. Immunohistochemistry confirmed the diagnosis of GIST, with CD117 (c-kit) positivity and CD34 negativity.
Given the high-risk nature of the tumor, the patient was referred for adjuvant therapy with imatinib, a tyrosine kinase inhibitor. Postoperative follow-up, including weekly monitoring for two months and subsequent monthly visits, showed no recurrence, and the patient remains under regular follow-up.
GIST are rare, mesenchymal neoplasms originating from the interstitial cells of Cajal, which are primarily found in the gastrointestinal tract. While they are more commonly observed in the stomach and small intestine, duodenal GISTs are exceedingly rare and account for only a small percentage of all GIST cases. Their clinical presentation is often nonspecific, with symptoms ranging from abdominal pain and gastrointestinal bleeding to more severe manifestations like bowel obstruction. In some cases, such as this one, the tumor may present with atypical symptoms such as obstructive jaundice, leading to diagnostic confusion and delays in identifying the true cause[1].
Historically, diagnosing GISTs relied heavily on clinical evaluation, endoscopy, and imaging techniques like computed tomography scans and magnetic resonance imaging. These imaging modalities have become essential in identifying tumors and assessing their size, location, and invasion of surrounding structures. However, even with advanced imaging, radiologic interpretation can be subjective, leading to challenges in distinguishing GISTs from other tumors with overlapping features, such as pancreatic cancer, neuroendocrine tumors, and lymphomas[5].
In this particular case, AI could have played a crucial role in improving the preoperative diagnosis. AI technologies, particularly machine learning algorithms and deep learning methods, have shown great potential in the analysis of medical images[6]. These AI tools are capable of identifying subtle and often imperceptible patterns in imaging data, which are critical for early detection and accurate diagnosis. For example, AI can evaluate tumor texture, morphology, vascularity, and peritumoral characteristics that human radiologists may overlook. These features are important in distinguishing GISTs from other tumors, especially in cases where the presentation is atypical[7].
In our case, the imaging findings suggested a periampullary mass that encased the common bile duct, pancreatic duct, and duodenum, leading to the clinical suspicion of pancreatic cancer. However, a machine learning model trained on imaging data from similar cases could have aided in distinguishing the duodenal GIST from pancreatic cancer, reducing the likelihood of misdiagnosis. AI-powered radiomics could have quantified the tumor’s characteristics, providing data on its heterogeneity and vascular involvement, which are known to correlate with the aggressiveness of GISTs[8]. This would have enabled a more precise diagnosis before surgery, guiding clinicians towards the correct course of treatment earlier.
One of the major challenges in diagnosing GISTs preoperatively is the need to differentiate them from other gastrointestinal tumors that exhibit similar clinical and radiological features[9]. Pancreatic cancer, neuroendocrine tumors, and lymphoma all have overlapping presentations with duodenal GISTs, making accurate preoperative diagnosis difficult. In the past, distinguishing between these conditions relied on subjective interpretation of imaging data, clinical judgment, and biopsy. However, AI has the potential to improve diagnostic accuracy by providing objective, data-driven insights[10]. Deep learning models that are trained on large datasets of medical images can automatically detect tumors, classify them, and even predict their malignancy with remarkable accuracy[11]. In the future, AI could be integrated into clinical practice as a decision-support tool, helping radiologists and surgeons make more informed, timely decisions[8,12].
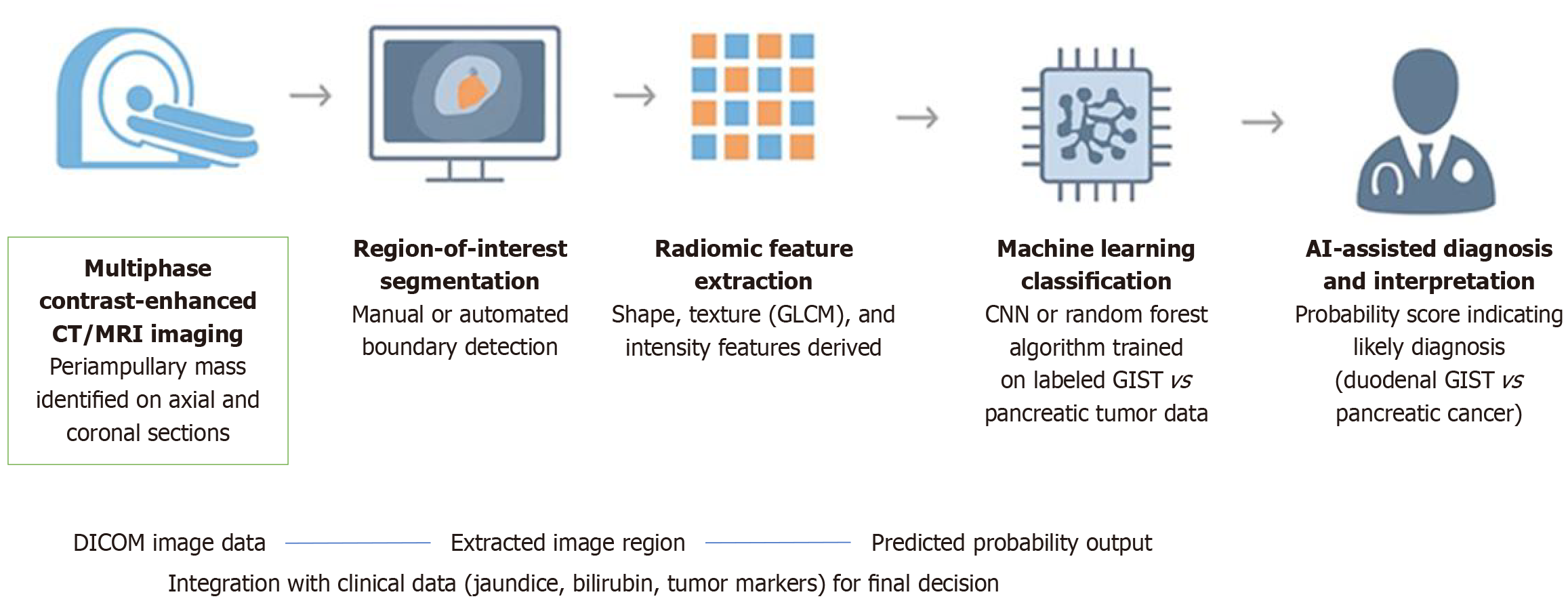
A practical illustration of how AI could have assisted in this case involves the application of radiomics-based texture analysis to the preoperative contrast-enhanced computed tomography images. Radiomics allows extraction of high-dimensional quantitative features - such as intensity, shape, and texture - from the tumor region of interest. These features are then processed using machine learning algorithms to identify unique imaging fingerprints that differentiate GISTs from other periampullary lesions. For instance, GISTs typically demonstrate higher entropy and surface irregularity compared to pancreatic adenocarcinomas, which often appear more homogeneous with ill-defined borders. AI models trained on annotated datasets could have recognized these subtle differences, suggesting the possibility of a submucosal duodenal lesion rather than a pancreatic primary.
In our hypothetical framework, a convolutional neural network could analyses multiphase computed tomography data to assess key parameters such as enhancement pattern, vascular encasement, and tumor origin. When combined with clinical data (age, bilirubin levels, and symptom profile), the AI model could output a probabilistic score indicating whether the lesion is more consistent with a GIST or a pancreatic carcinoma. This approach, if applied preoperatively, might have prompted a targeted endoscopic biopsy or modified surgical planning, reducing the risk of overtreatment with a pancreaticoduodenectomy.
AI in radiomics extracts a large volume of quantitative data from medical images, which can then be analyzed to provide information about tumor heterogeneity - a key feature that differentiates GISTs from other gastrointestinal tumors. Radiomic features such as tumor texture, shape irregularity, entropy, and gray-level co-occurrence matrix values could help in predicting the biological behavior of the tumor, including its likelihood of recurrence or metastasis. For instance, higher heterogeneity in GISTs has been associated with more aggressive tumors. AI tools could have detected these features early, predicting a high-risk tumor and prompting earlier intervention with adjuvant therapy[7].
Recent studies have demonstrated that AI-assisted image classifiers can achieve diagnostic accuracies exceeding 90% in differentiating gastrointestinal stromal tumors from other mesenchymal and epithelial lesions. For example, Li et al[8] developed a deep-learning model using microprobe endoscopic ultrasonography that accurately identified GIST characteristics, while Dong et al[13] validated a real-time AI system for discriminating GISTs from leiomyomas with high sensitivity and specificity. These findings underscore AI’s growing potential as a decision-support tool in complex diagnostic settings. Furthermore, radiogenomic integration - linking imaging features with mutational profiles (e.g., KIT or platelet-derived growth factor gene mutations) - could further refine risk prediction and therapeutic planning for patients with GIST.
While AI holds transformative potential, its application in rare tumor diagnosis faces several challenges. Dataset scarcity limits algorithmic generalizability, as models trained on common tumors may underperform on rare variants such as duodenal GISTs. Ethical issues also arise concerning data privacy, algorithmic transparency, and the risk of overreliance on automated systems. Thus, AI tools should complement, not replace, expert clinical judgment. Future multicenter collaborations aimed at creating balanced datasets and explainable AI models are essential to ensure equitable and clinically reliable deployment of such technologies in diagnostic radiology.
Moreover, AI’s ability to integrate multi-modal data, such as clinical records, radiologic images, and genomic profiles, presents an opportunity for more comprehensive diagnostic pathways. By combining these datasets, AI could help identify molecular signatures of GISTs - such as KIT gene mutations - allowing for a diagnosis even before histopathological results are available[14]. For example, integrating genomic data with imaging features could enhance the predictive accuracy of AI models, providing not only a diagnostic confirmation but also an estimate of tumor behavior based on genetic profiling[8,12].
The proposed workflow begins with the acquisition of high-resolution multiphase contrast-enhanced computed tomo
Shape features: Surface area-to-volume ratio, compactness, and sphericity.
Texture features: Entropy, gray-level co-occurrence matrix contrast, and uniformity.
Intensity features: First-order histogram metrics and enhancement variability across arterial and venous phases.
These quantitative parameters are fed into a supervised machine learning model trained on labelled datasets of duodenal GISTs and pancreatic tumors. The output probability assists clinicians in classifying the lesion type and guiding preoperative decision-making. The framework exemplifies how AI could have influenced our case by suggesting a duodenal origin and prompting confirmatory endoscopic biopsy before major surgery.
Furthermore, AI’s role in predicting patient outcomes should not be underestimated. In this case, adjuvant therapy with imatinib was essential due to the tumor’s high mitotic rate, which posed a risk of recurrence[15]. AI models can be designed to analyses preoperative imaging and predict the tumor’s response to treatment. By identifying characteristics like tumor vascularity, necrosis, and proliferation rate, AI could predict how well the tumor will respond to tyrosine kinase inhibitors like imatinib, helping clinicians tailor the therapy to achieve the best possible outcome[8,12].
AI could also improve the overall efficiency of the diagnostic pathway. By automating certain aspects of the diagnostic process, AI could reduce the time needed to assess imaging data, allowing for quicker decision-making and earlier intervention[5,16,17]. For instance, AI could instantly provide a risk classification of the tumor, flagging high-risk cases for immediate biopsy or surgical intervention. This could have been particularly useful in this case, where the tumor’s high mitotic activity warranted urgent adjuvant therapy[13].
Lastly, the integration of AI into the clinical workflow would enhance multidisciplinary collaboration. AI tools can provide actionable insights that can be easily shared among oncologists, radiologists, surgeons, and pathologists, fostering better communication and a more coordinated treatment plan. As AI technologies evolve, they will continue to improve the precision and timeliness of diagnosis and treatment, potentially reducing the overall morbidity and mortality associated with rare and complex conditions like duodenal GISTs[18].
AI can enhance the preoperative diagnosis of duodenal GISTs by detecting subtle features in imaging data that distinguish them from other gastrointestinal malignancies. AI-powered radiomics and deep learning algorithms improve diagnostic accuracy by identifying tumor heterogeneity and predicting tumor behavior. Integrating AI into clinical workflows can accelerate diagnosis, improve treatment planning, and potentially lead to better patient outcomes. The preoperative use of AI could have aided in a more accurate diagnosis and earlier intervention, improving the clinical outcome for patients with rare tumors like duodenal GISTs. Regular integration of AI in radiological imaging and multidisciplinary collaboration will help streamline decision-making processes and enhance the quality of care for rare gastrointestinal malignancies (Table 1).
| Ref. | Year | Presenting symptoms | Key findings |
| Shanker et al[1] | 2024 | GI bleeding, obstructive jaundice | GISTs are rare, accounting for 3%-5% of duodenal cases. GISTs can present with symptoms like GI bleeding and jaundice |
| Yang et al[18] | 2025 | Fatigue, melena | GISTs in children are often asymptomatic but can present with fatal GI bleeding; initial misdiagnosis can occur |
| Amin et al[14] | 2024 | Melena, anemia | A duodenal GIST mimicking other conditions, emphasizes importance of considering GIST in differential diagnosis of duodenal lesions |
| Marques-Antunes et al[17] | 2024 | Not specified | Pathological complete response after neoadjuvant imatinib therapy in recurrent duodenal GIST, highlighting molecular testing |
| Fournier et al[15] | 2024 | Duodenal mass | Duodenal GNET can mimic GISTs; early recognition is essential due to poor prognosis and its ability to metastasize |
In this case, a duodenal GIST was initially misdiagnosed as pancreatic cancer due to its presentation with obstructive jaundice and similar imaging characteristics. The definitive diagnosis was only made postoperatively through histopathological examination, highlighting the challenges in diagnosing rare tumors like GISTs. However, AI could have significantly improved the preoperative diagnostic process by analyzing computed tomography scan images and identifying features suggestive of a GIST, helping clinicians make a more accurate diagnosis before surgery.
By using AI-enhanced imaging analysis and radiomic features, clinicians could have better distinguished the duodenal GIST from pancreatic cancer, reducing diagnostic errors and enabling more targeted treatment decisions. AI could have also provided valuable insights into the tumor’s behavior, such as its aggressiveness and response to treatment, helping guide adjuvant therapy decisions, such as the use of imatinib. As AI continues to evolve, it is poised to play a critical role in transforming the diagnostic pathway for rare cancers like duodenal GISTs, improving early detection, treatment outcomes, and patient survival. The integration of AI into clinical practice holds tremendous promise for enhancing diagnostic accuracy, improving patient care, and reducing the risk of misdiagnosis in challenging cases.
| 1. | Shanker DA, Kumar S, Al-Mukhtar A, Dube A, Samuel N. A Case of Gastrointestinal Stromal Tumour (GIST) in the Duodenum in a Young Adult. Cureus. 2024;16:e53331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Wakebe M, Kawamoto S, Sasaki T, Mikajiri Y, Yamamoto K, Terashima T, Kurogi N. [A Case of Pancreaticoduodenectomy Performed after Chemotherapy and Arterial Embolization for a Giant Duodenal GIST in a Jehovah's Witness]. Gan To Kagaku Ryoho. 2024;51:1561-1563. [PubMed] |
| 3. | Zheng L, Jin DW, Yu HW, Yu Z, Qian LY. Application of AI in the identification of gastrointestinal stromal tumors: a comprehensive analysis based on pathological, radiological, and genetic variation features. Front Genet. 2025;16:1555744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Lu Y, Wu J, Hu M, Zhong Q, Er L, Shi H, Cheng W, Chen K, Liu Y, Qiu B, Xu Q, Lai G, Wang Y, Luo Y, Mu J, Zhang W, Zhi M, Sun J. Artificial Intelligence in the Prediction of Gastrointestinal Stromal Tumors on Endoscopic Ultrasonography Images: Development, Validation and Comparison with Endosonographers. Gut Liver. 2023;17:874-883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Shah B, Bajaj J, Yadav DS, Mahajan C, Vijendra AR. Ruptured Duodenal GIST in a Young Female - A Rare Presentation and Comprehensive Review. Ann Afr Med. 2024;23:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Zhuo M, Chen X, Tang Y, Guo J, Tang X, Qian Q, Xue E, Chen Z. Use of a Convolutional Neural Network to Predict the Malignant Potential of Gastrointestinal Stromal Tumors in Transabdominal Ultrasound Images: Visualization of the Focus of the Prediction Model. Ultrasound Med Biol. 2023;49:1951-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Joo DC, Kim GH, Lee MW, Lee BE, Kim JW, Kim KB. Artificial Intelligence-Based Diagnosis of Gastric Mesenchymal Tumors Using Digital Endosonography Image Analysis. J Clin Med. 2024;13:3725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Li J, Jing X, Zhang Q, Wang X, Wang L, Shan J, Zhou Z, Jiang D, Yan Y, Liu L, Zhao M, Fan L, Zheng C, Gong X, Sun X. Interpretable deep learning model diagnoses gastrointestinal stromal tumors and lesion characteristics with microprobe endoscopic ultrasonography. Sci Rep. 2025;15:34366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Yadav SK, Bhattarai HB, Rijal A, Shrestha A, Shah S, Subedi A, Yadav BK, Acharya A, Khatri R, Kadel G. Duodenal gastrointestinal stromal tumor: A case report. Ann Med Surg (Lond). 2022;82:104574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Varshney VK, Nayar R, Yadav T, Khera S. Duodenal gastrointestinal stromal tumour imitating as pancreatic head tumour. BMJ Case Rep. 2022;15:e248828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Lüthje P, Nurmi-Lüthje I. Incidentally Discovered Duodenal Gastrointestinal Stromal Tumour (GIST): Operative Treatment and Problems After Surgery-A Case Report and Literature Review. Case Rep Gastrointest Med. 2025;2025:5493240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Alghafees M, Seyam RM, Al-Hussain T, Amin TM, Altaweel W, Sabbah BN, Sabbah AN, Almesned R, Alessa L. Using machine learning models to predict synchronous genitourinary cancers among gastrointestinal stromal tumor patients. Urol Ann. 2024;16:94-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Dong Z, Zhao X, Zheng H, Zheng H, Chen D, Cao J, Xiao Z, Sun Y, Zhuang Q, Wu S, Xia J, Ning M, Qin B, Zhou H, Bao J, Wan X. Efficacy of real-time artificial intelligence-aid endoscopic ultrasonography diagnostic system in discriminating gastrointestinal stromal tumors and leiomyomas: a multicenter diagnostic study. EClinicalMedicine. 2024;73:102656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 14. | Amin M, Nageeb A, Abuhashem S, Saleh A, Awad E, Raed R. Common Symptoms and a Rare Diagnosis: A Case of Duodenal Gastrointestinal Stromal Tumor Presenting as Gastrointestinal Bleeding. Cureus. 2024;16:e69814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Fournier A, Deslauriers V, Giguère CC, Borduas M, Collin Y. Malignant duodenal gastrointestinal neuroectodermal tumor (GNET): Case report and review of the literature. Int J Surg Case Rep. 2024;123:110195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Kim Y, Lee SH. Pathologic diagnosis and molecular features of gastrointestinal stromal tumors: a mini-review. Front Oncol. 2024;14:1487467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Marques-Antunes J, Carvalho L, Pereira S, Ferreira T, Nora M. Pathological Complete Response After Neoadjuvant Imatinib in a Recurrent Duodenal Gastrointestinal Stromal Tumor (GIST). Cureus. 2024;16:e64669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Yang C, Li K, Xiang B. Case Report: Duodenal gastrointestinal stromal tumor misdiagnosed as tumor located on the major duodenal papilla leading to fatal gastrointestinal bleeding in a child. Front Pediatr. 2025;13:1546914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/